Method for producing liquid of barium chloride by using mineral powder of Duyingshi in low grade
A technology of witherite and low-grade is applied in the field of producing liquid barium chloride from low-grade witherite ore powder, which can solve the problem of high production cost, achieve good economic and environmental benefits, and avoid the effects of environmental pollution.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
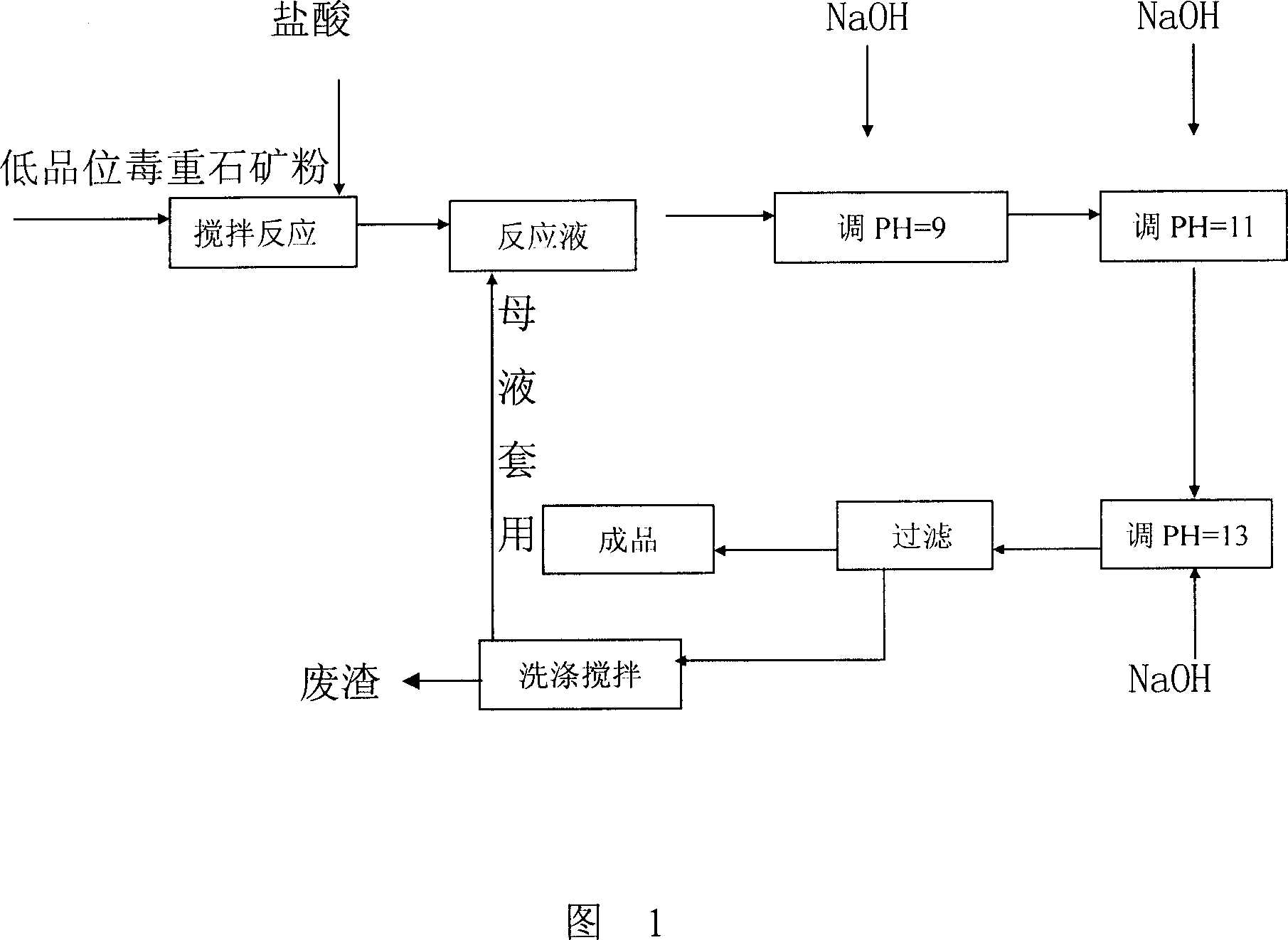
[0008] Embodiment 1: 3000Kg of witherite powder with a content of 68% is dropped into the reaction tank, and the hydrochloric acid with a concentration of 30% is added, stirred while adding, and the reaction is completed in 3 hours. When the value is 4-5, continue to stir for 30 minutes, add a NaOH solution with a concentration of 42%, and adjust the pH value to 9. At this time, some Ca 2+ Precipitation occurs, Ca 2+ +2OH→Ca(OH) 2 ↓. Stir for 20 minutes, then add concentration and be 42% NaOH, adjust pH value to be 11, this moment Ca 2+ , Mg 2+ The precipitation is basically completed, and there is a small amount of Sr 2+ Precipitate, stir for 20 minutes, then add NaOH solution with a concentration of 42%, adjust the pH value to 13, and now there are most of the Sr 2+ Precipitation, Sr 2+ +2OH→Sr(OH)↓After stirring for 20 minutes, the reaction solution is pumped into the plate and frame filter press, the clear liquid is the product, the filter cake is washed and filtered...
Embodiment 2
[0010] Embodiment 2: 3200Kg of witherite powder with a content of 68% is dropped into the reaction tank, and a concentration of 33.7% hydrochloric acid is added, stirred while adding, and the reaction is completed in 3 hours. When the value is 4-5, continue to stir for 30 minutes, add a concentration of 32% NaOH solution, adjust the pH value to 9, the following process is the same as Example 1, and the concentration of NaOH solution used is 32%. The results of the serum test are as follows:
[0011] BaCl 2 209.9g / l Mg 2+ +Ca 2+ 13.69g / l.Sr 2+ 0.75g / l.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com