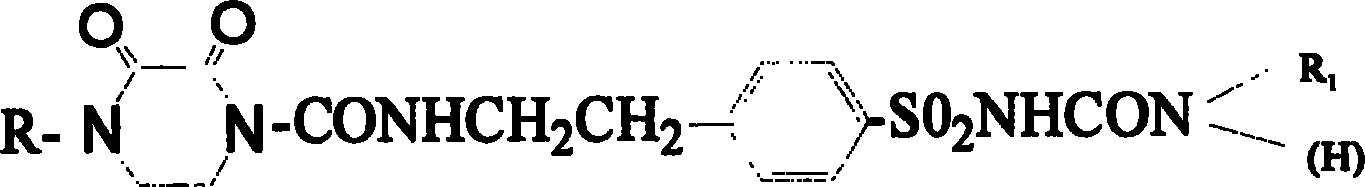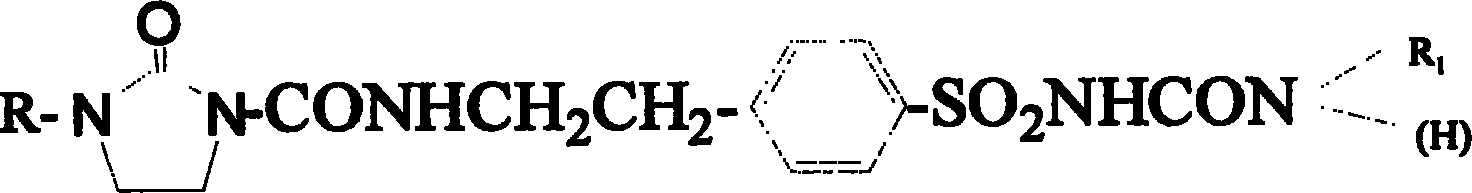New compound in sulfonyl ureas, and medicine use
A kind of compound and drug technology, applied in the field of new sulfonylurea compound and its medical use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Preparation of 1-{4-[2-(1-ethyl-2,3-dioxopiperazine-4-carboxamido)ethyl]benzenesulfonyl}-3-cyclohexylurea (code PHN)
[0025] Put 1.84 grams of 4-[2-(1-ethyl-2,3-dioxopiperazine-4-formamido)-ethyl]benzenesulfonamide, 30ml of anhydrous acetonitrile, and 1.38 grams of anhydrous potassium carbonate In a 100ml round bottom flask, heat to reflux on an oil bath and stir for 1h. Then slowly add 0.81 g of cyclohexyl isocyanate in acetonitrile solution dropwise. After the addition, continue to stir and reflux for 6 hours, then cool and filter. The filter cake was dissolved with 30 ml of ice water, filtered, and the filtrate was acidified to pH 1 with hydrochloric acid, and crude PHN was precipitated. The crude product was recrystallized from dilute methanol to obtain 1.76 g of white solid. Yield 68.22%.
[0026] 1 H NMR (400MHz, DMSO-d 6 )
[0027] δ=1.058 (triplet, J=7.28Hz, 3H); 1.114 (multiplet, J=9.80Hz, 4H); 1.468 (multiplet, 2H); 1.566 (multiplet, J=3.36Hz, 4H); 2.88...
Embodiment 2
[0032] 1-{4-[2-(1-Ethyl-2,3-dioxopiperazine-4-carboxamido)ethyl]benzenesulfonyl}-3-(trans-4-methylcyclohexyl) Preparation of urea (code PJN)
[0033] PJN was prepared according to Example 1. Yield 60.22%.
[0034] PJN molecular formula and molecular weight C 23 h 33 N 5 o 6 S(507.6)
[0035] 1 H NMR (400MHz, DMSO-d 6 )
[0036] δ=0.815 (doublet, J=6.44Hz, 3H); 0.870 (triplet, J=12.32Hz, 3H); 1.072 (multiplet, J=3.08Hz, 4H); 1.238 (multiplet, J=5.32Hz , 1H); 1.583 (doublet, J=12.60Hz, 2H); 1.668 (doublet, J=12.04Hz, 2H); 2.725 (triplet, J=7.00Hz, 2H); 2.887 (triplet, J=7.00Hz, 2H); 7.00Hz, 2H); 3.231 (complex peak, 1H); 3.493 (triplet, J=7.00Hz, 2H); 3.533 (quartet, J=5.88Hz, 2H); 3.904 (complex peak, 2H); 6.279 (doublet, J=7.28Hz, 1H); 7.453 (doublet, J=8.12Hz, 2H); 7.805 (doublet, J=8.40Hz, 2H); 8.905 (triplet, J=5.60Hz, 1H) ; 10.336 (singlet, 1H).
[0037] MS(FAB): m / z=508.3[MH]+
Embodiment 3
[0039] Preparation of 1-{4-[2-(1-acetyl-2-oxo-imidazolidinone-3-carboxamido)ethyl]benzenesulfonyl}-3-phenylethylurea (code MBN)
[0040] 1.77 grams (0.005mol) of 4-[2-(1-acetyl-2-oxo-imidazolidinone-3-carboxamido)-ethyl]benzenesulfonamide, 30ml of anhydrous acetone, 1.38 grams (0.01mol ) Anhydrous potassium carbonate was placed in a 100ml round bottom flask, heated to reflux on an oil bath, and stirred for 1h. Then slowly add 0.81 g (0.0055 mol) of an acetone solution of phenylethyl isocyanate dropwise, after the addition, continue to stir and reflux for 6 hours, cool, filter, dissolve the filter cake with 30 ml of ice water, filter, and use hydrochloric acid to dissolve the filter cake Acidify to pH 1, collect the solid by filtration, and dry to obtain crude MBN, which is recrystallized from dilute methanol to obtain 1.68 g of white solid. Yield 66.93%.
[0041] 1 H NMR (400MHz, DMSO-d 6 )
[0042]δ=2.623 (triplet, J=7.28Hz, 2H); 2.750 (triplet, J=7.00Hz, 2H); 2.837 (tri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com