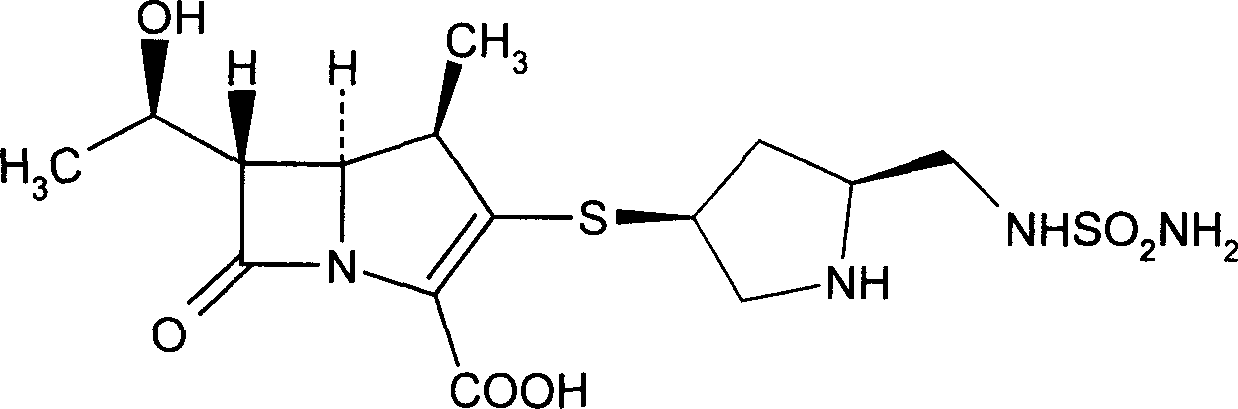
Doriipenem hydrate crystal and preparation method thereof
A hydrate and crystallization technology, which is applied in the direction of pharmaceutical formulations, medical preparations containing active ingredients, antibacterial drugs, etc., can solve the problems of poor crystal stability of doripenem, complicated preparation methods, time-consuming and energy-consuming, etc., and achieve excellent The effects of stability, simple preparation method, and excellent solubility properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] The crude product of doripenem (10 g) was dissolved in distilled water (200 mL) at 50-55°C, added with activated carbon (0.6 g) for decolorization, and suction filtered. Add isopropanol (90 mL) to the filtrate while it was hot, cool to 15-20°C and stir for 1 hour, then cool to 0-5°C and stir for 2 hours. Isopropanol (90 mL) was added dropwise to the resulting suspension, then stirred at 0-5°C for 2 hours, and finally cooled to -10°C and stirred overnight. The precipitated crystals were collected by filtration, and the filter cake was washed with isopropanol:water (volume ratio 4:1). The resulting filter cake was dried at 50° C. under reduced pressure (0-10 mmHg) for about 4 hours until the water content was about 6%, and the doripenem type V crystallized.
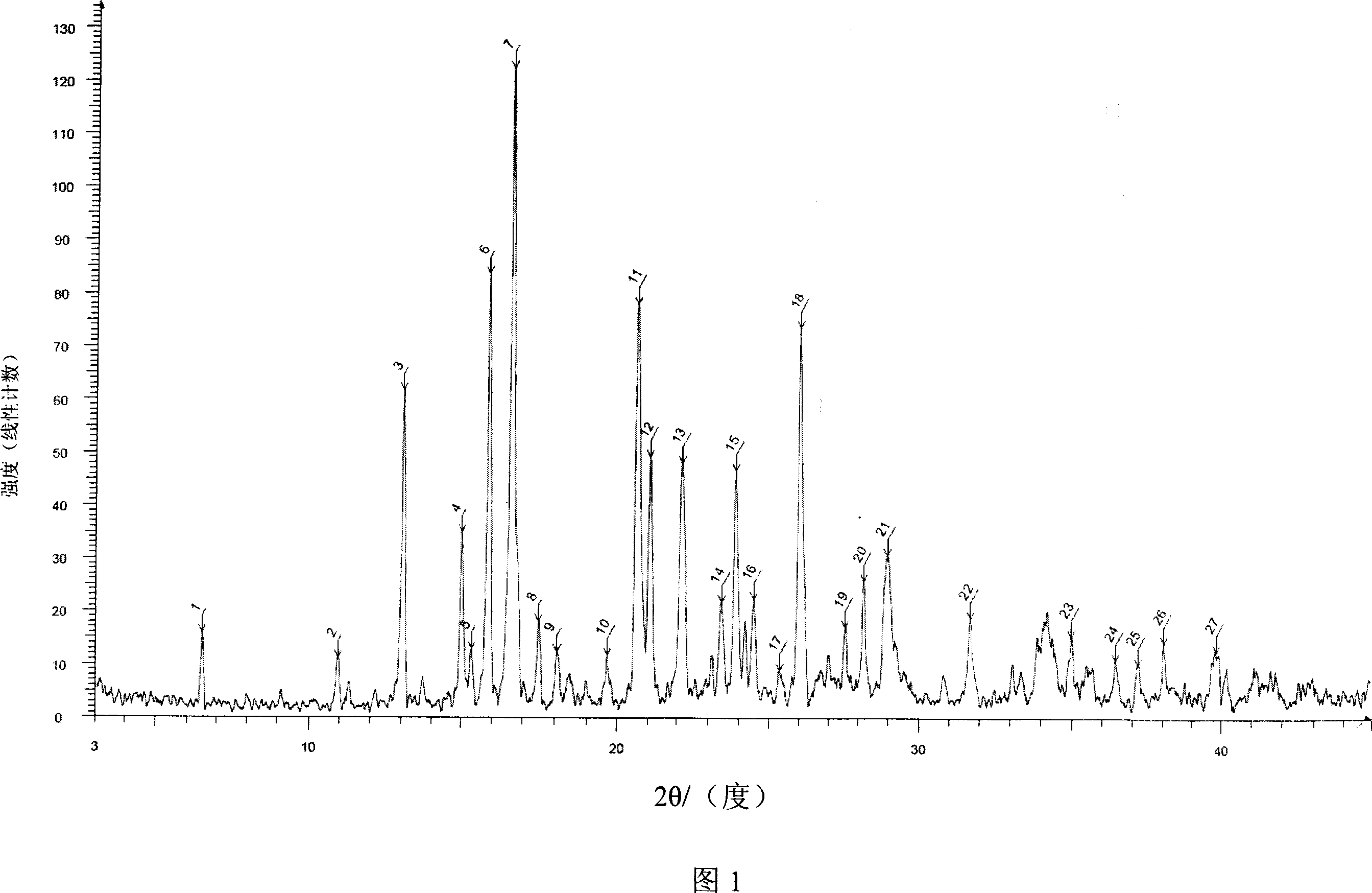
[0044] The powder X-ray diffraction spectrum of the obtained crystal is shown in Fig. 1, wherein at diffraction angles 2θ=6.45, 13.05, 14.99, 15.31, 15.89, 16.64, 17.50, 18.07, 19.67, 20.65, 21.07, 22.17, 23.44, 23....
Embodiment 2
[0050] The crude product of doripenem (10 g) was dissolved in distilled water (100 mL) at 60-65°C, added with activated carbon (0.3 g) for decolorization, and suction filtered. The filtrate was cooled to room temperature, stirred for 1 hour, then cooled to 0-5°C and stirred for 3 hours; isopropanol (100 mL) was added dropwise to the obtained suspension, and then stirred at 0-5°C for 2 hours. Finally, it was cooled to -15°C and stirred overnight, the precipitated crystals were collected by filtration, and the filter cake was washed with isopropanol:water (volume ratio 4:1). The resulting filter cake was dried at 50° C. under reduced pressure (10-20 mmHg) for about 5.5 hours until the water content was about 6%, and doripenem type VI crystallized.
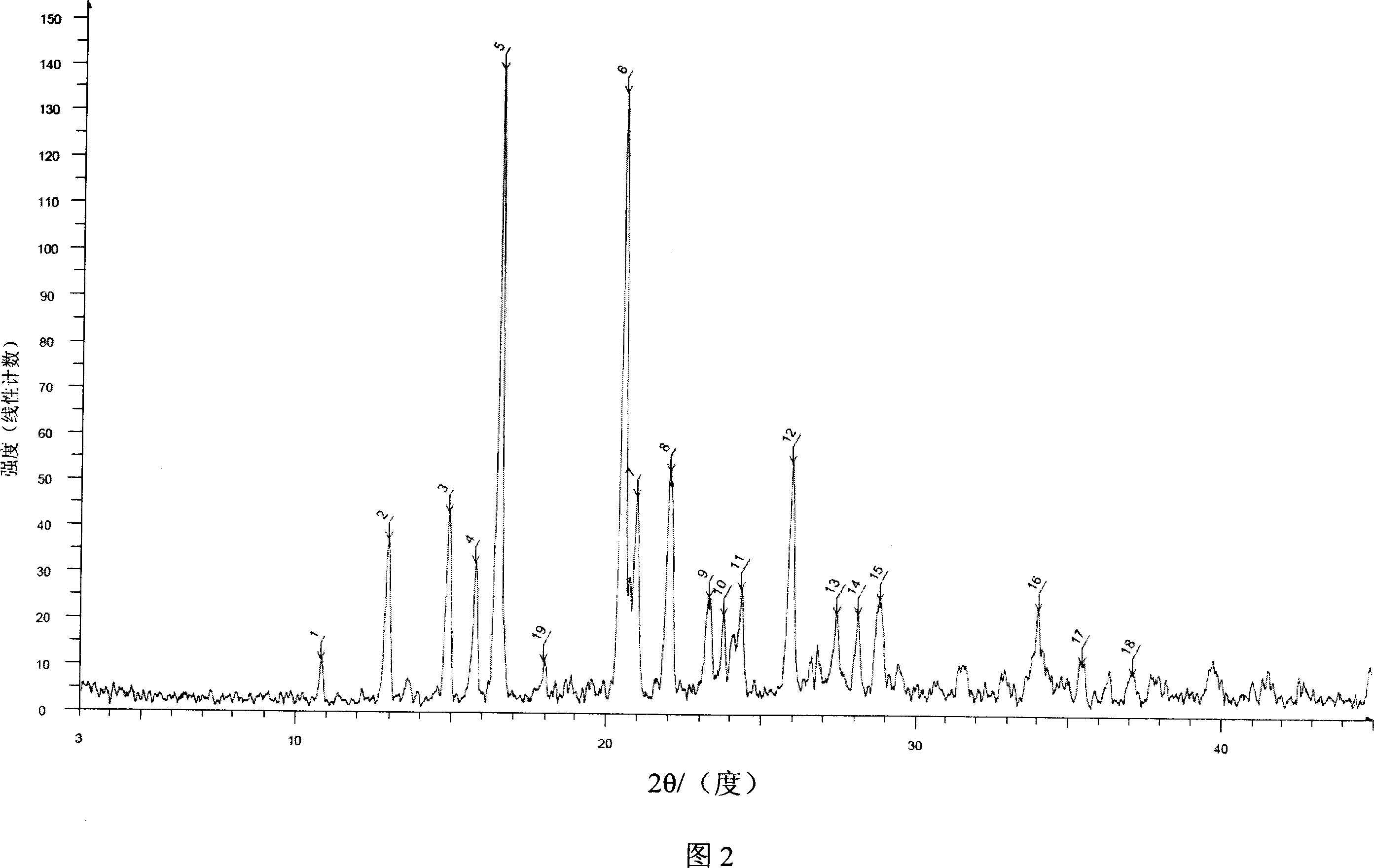
[0051] The powder X-ray diffraction spectrum of the resulting crystal is shown in Figure 2, wherein there are main peaks at diffraction angles 2θ=12.91, 14.87, 15.75, 16.52, 20.52, 20.97, 22.05, 23.31, 23.79, 24.37, 25.98, 27.43, 28....
Embodiment 3
[0058] The crude product of doripenem (10 g) was dissolved in distilled water (200 mL) at 50-55°C, added with activated carbon (0.6 g) for decolorization, and suction filtered. Add isopropanol (90 mL) to the filtrate while it is hot, put doripenem type V crystal seed crystals into the resulting solution, cool to 15-20°C and stir for 1 hour, then cool to 0-5°C and stir for 2 hours. Isopropanol (90 mL) was added dropwise to the resulting suspension, then stirred at 0-5°C for 2 hours, and finally cooled to -10°C and stirred overnight. The precipitated crystals were collected by filtration, and the filter cake was washed with isopropanol:water (volume ratio 4:1). The resulting filter cake was dried at 50°C under reduced pressure (0-10mmHg) for about 4 hours to a water content of about 6%, and doripenem type V crystals (8.4g, purification rate 84%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com