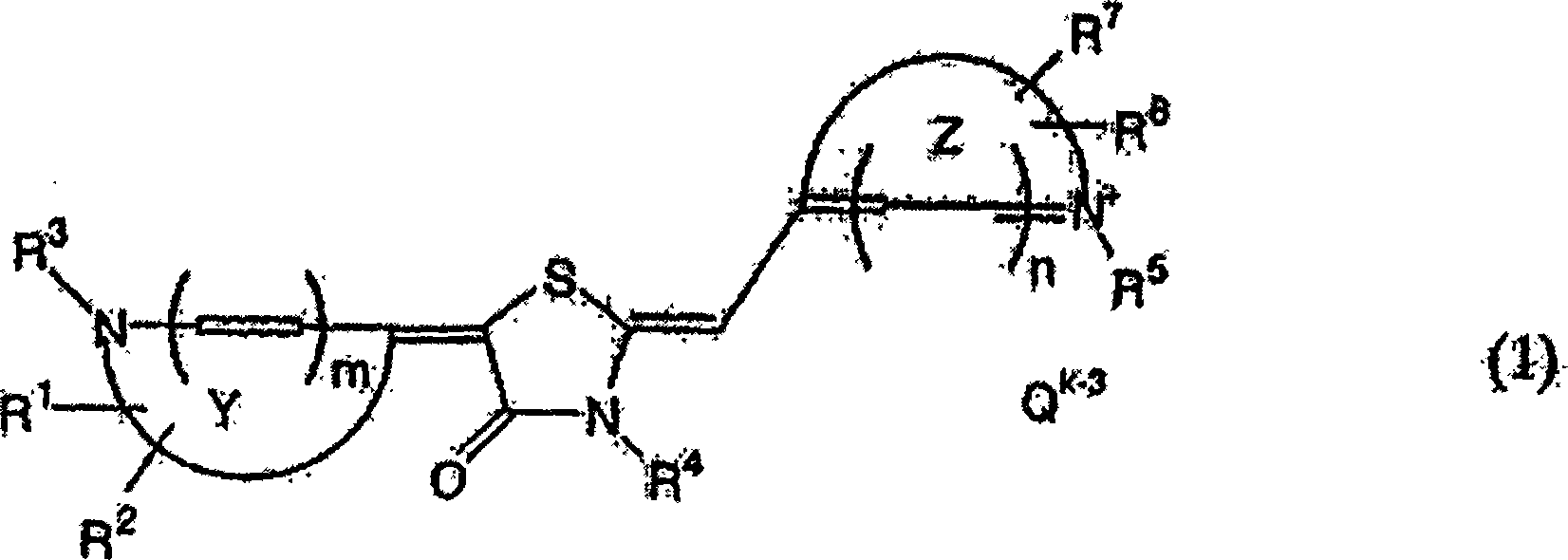
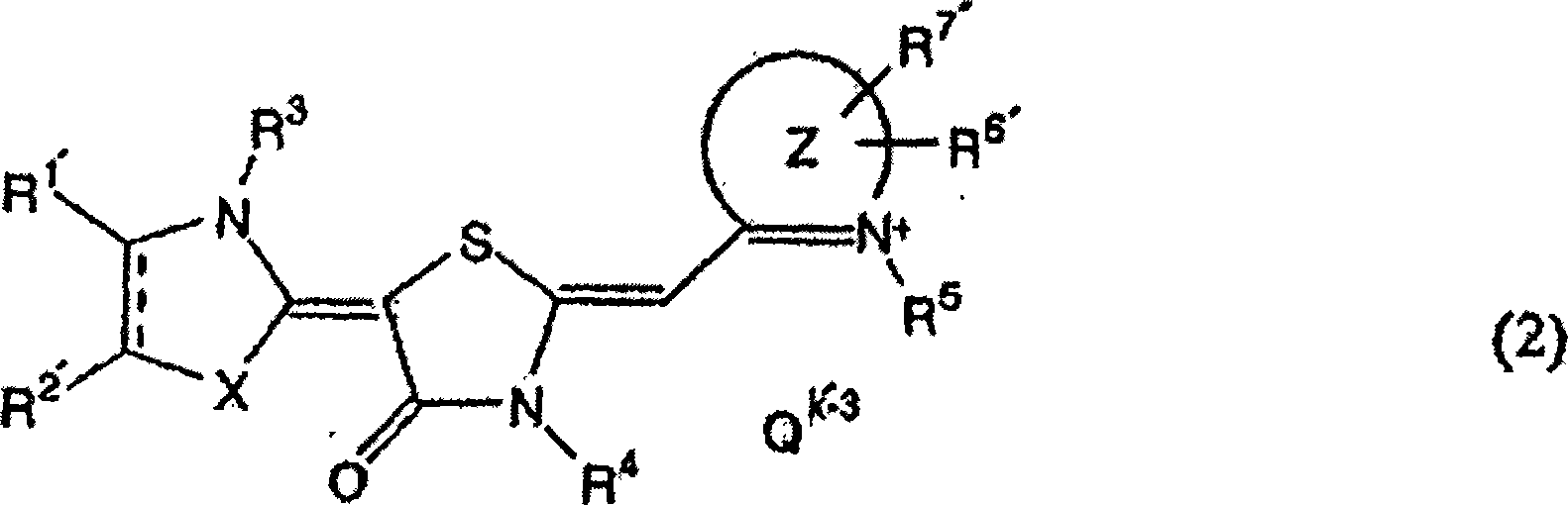
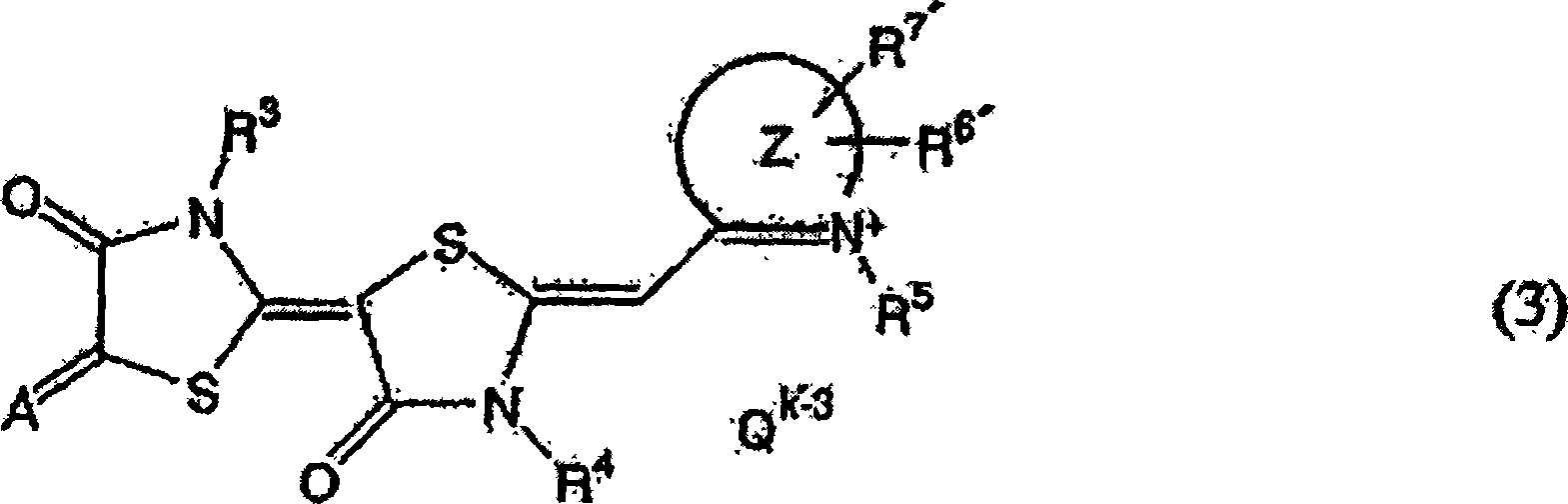
Anti-trypanosomiasis agent
An anti-trypanosomiasis and trypanosomiasis technology, applied in the direction of resistance to vector-borne diseases, anti-infective drugs, drug combinations, etc., can solve the problems of heavy physical and economic burden, harmful side effects, and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] 1-1. Culture of African trypanosomiasis protozoa
[0069] In this example, the bloodstream-dwelling trypanomastigotes of the protozoa of Trypanosoma brucei rhodensiense (STIB900 strain) were used. The medium used in the experiment was filter-sterilized MEM medium supplemented with 25mM N-2-hydroxyethylpiperazine-2-ethanesulfonic acid (HEPES), 1g / L glucose, 1% MEM non- Essential amino acids, 0.2 mM 2-mercaptoethanol, 2 mM sodium pyruvate, 0.1 mM hypoxanthine, and 15% heat-treated horse serum. Protozoa were cultured in air with a CO2 concentration of 5% at a temperature of 37°C.
[0070] 1-2. African trypanosomiasis protozoan proliferation inhibition screening test
[0071] The compound of the present invention used in the test and the positive drug (Melarsoprol (Melarsoprol)) were dissolved in dimethyl sulfoxide (DMSO) to prepare a test solution with a predetermined concentration. Add the medium containing 8×103 protozoa and the test solution containing the drug at a ...
Embodiment 2
[0092] 2-1. Culture of Chagas disease protozoa
[0093] In this example, amastigotes and trypanomastigotes of the protozoa of Trypanosoma cruzi (Tulahuen C2C4 strain) infected with rat L6 cells were used. The medium used in the experiment was RPMI1640 medium containing L6 cells with L-glutamine (200 mM) added at a final concentration of 1%, fetal bovine serum at a final concentration of 10%, and cultured at 37°C.
[0094] 2-2. Screening test for protozoan proliferation inhibition of American trypanosomiasis
[0095] The compound of the present invention and the positive drug (Benznidazol) used in the test were dissolved in DMSO to prepare a test solution with a predetermined concentration. A culture medium containing 5×10 3 protozoa was added to the wells of a 96-well culture plate, and pre-cultured for 48 hours. After exchanging the medium, a test solution containing a drug at a predetermined concentration or DMSO without a drug was added. Divide the test solution into two...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com