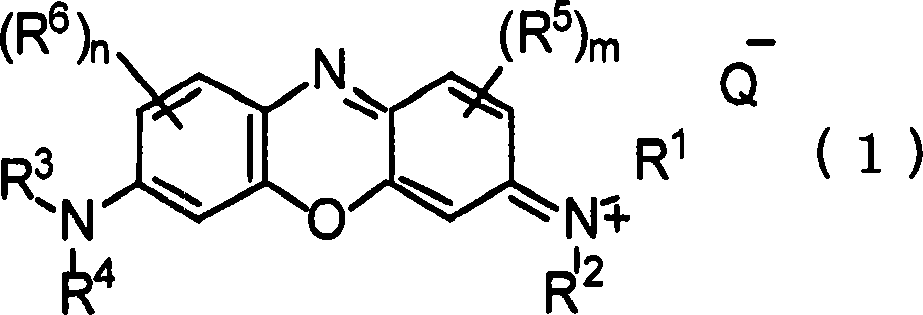
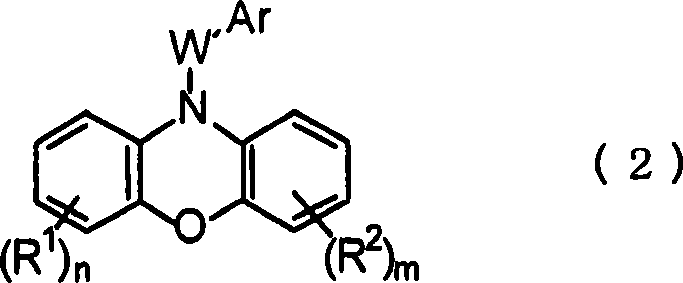
Pharmaceutical composition containing phenoxazinium compound as active ingredient
A technology for active ingredients and compositions, which is applied in the field of pharmaceutical compositions containing phenoxazine compounds as active ingredients, can solve the problems of high acute toxicity, difficult therapeutic effect, and is not suitable for large-scale administration, and achieves remarkable curative effect, Effects of high selective toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
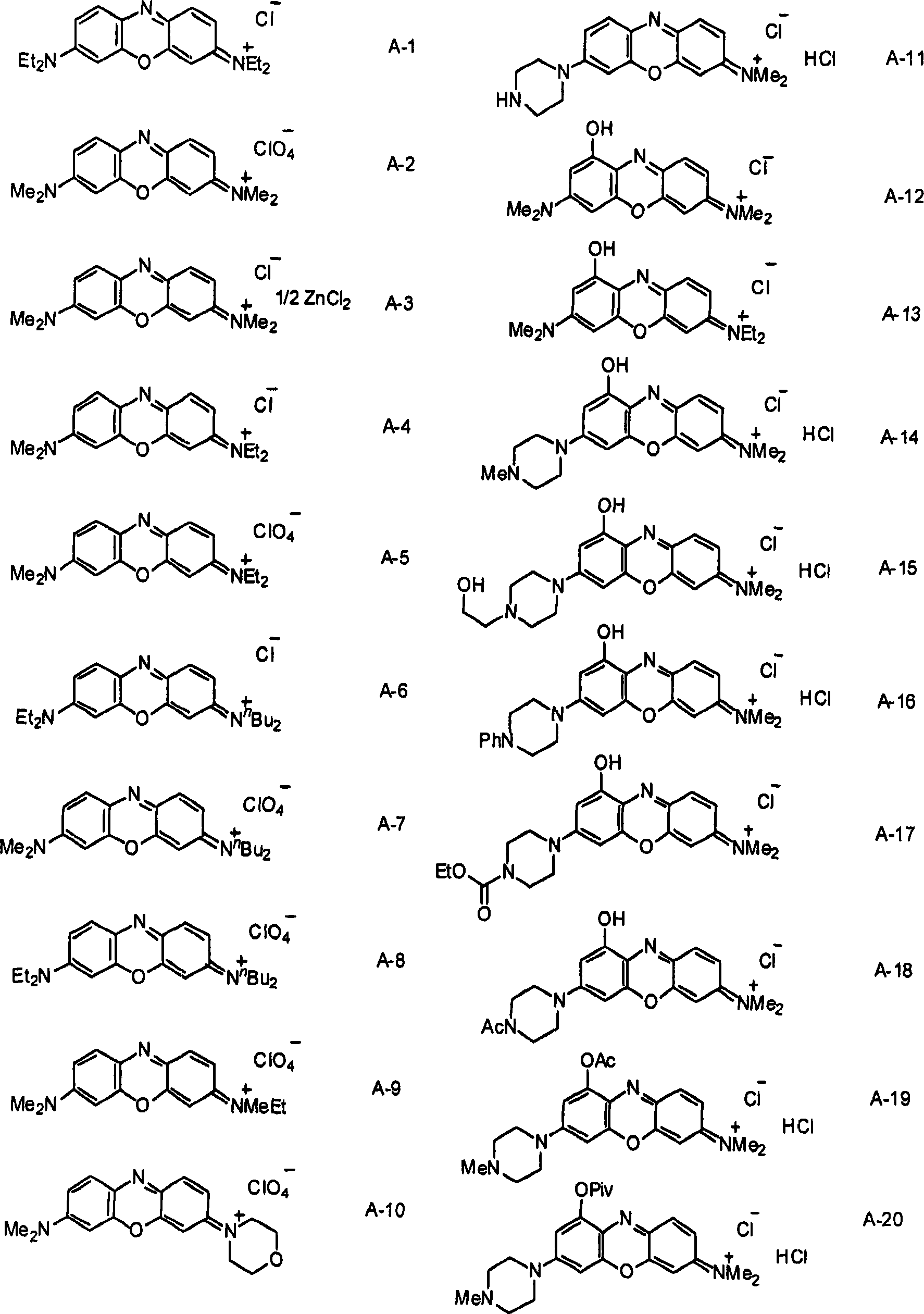
[0072] Synthesis of 3-Dibutylamino-7-diethylaminophenoxazinium Perchlorate (Compound A-8)
[0073] A mixture of 3-butylaminophenol (1.0 mL, 4.43 mmol) and N,N-diethyl-4-nitrosoaniline (790 mg, 4.43 mmol) was suspended in ethanol (55 mL) at room temperature, and 60% aqueous perchloric acid (0.5 mL) was added dropwise to the suspension. The resulting mixture was heated to reflux for 3 hours and cooled to room temperature. It was then concentrated under reduced pressure to half its starting volume of solvent and cooled to 0 °C. The resulting precipitate was removed by filtration and the filtrate was concentrated. The concentrated crude material was purified by silica gel chromatography (eluent: chloroform:ethyl acetate=9:1) to obtain a crude compound. The resulting crude compound was dissolved in methanol and cooled to 0°C, and a few drops of diethyl ether were added thereto for crystallization. The obtained dark blue crystals were filtered to obtain 3-dibutylamino-7-diethy...
Embodiment 2
[0076] Synthesis of 3-ethylmethylamino-7-dimethylaminophenoxazinium perchlorate (compound A-9)
[0077] A mixture of 3-methylethylaminophenol (300 mg, 1.98 mmol) and N,N-dimethyl-4-nitrosoaniline (298 mg, 1.98 mmol) was suspended in ethanol (15 mL) at room temperature, And 70% aqueous perchloric acid (0.5 mL) was added dropwise to the suspension. The resulting mixture was heated to reflux for 6 hours, cooled to room temperature and distilled under reduced pressure to remove the solvent. The resulting crude material was purified by silica gel chromatography (eluent: chloroform:ethyl acetate=9:1) to obtain a crude compound (216 mg, crude yield 27%). The resulting crude compound was dissolved in methanol and cooled to 0°C for crystallization. The resulting dark blue crystals were filtered to obtain 3-ethylmethylamino-7-dimethylaminophenoxazinium perchlorate (13.3 mg, isolated yield 2%).
[0078] 1H-NMR (400MHz, CDCl3) δ: 7.80(s, 1H), 7.78(s, 1H), 7.41(dd, 1H, J=6.8, 2.8Hz), ...
Embodiment 3
[0080] Synthesis of 3-dimethylamino-7-(1-piperazinyl) phenoxazinium chloride monohydrochloride (compound A-11) become
[0081]A mixture of 3-(1-piperazinyl)phenol (165 mg, 0.92 mmol) and N,N-dimethyl-4-nitrosoaniline (139 mg, 0.92 mmol) was suspended in ethanol (50 mL) at room temperature , and 70% aqueous perchloric acid (0.5 mL) was added dropwise to the suspension. The resulting mixture was heated to reflux for 6 hours, cooled to room temperature and distilled under reduced pressure to remove the solvent. The obtained crude material was purified by silica gel chromatography (eluent: chloroform:ethyl acetate=9:1) to obtain a crude compound (65 mg). The obtained crude compound was dissolved in methanol, mixed with ion exchange resin Amberlyte IRA-400 (C1) and left at room temperature for 2 hours. The resulting mixture was then filtered, and the resin was further washed with methanol. The collected filtrate was concentrated under reduced pressure for crystallization. T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com