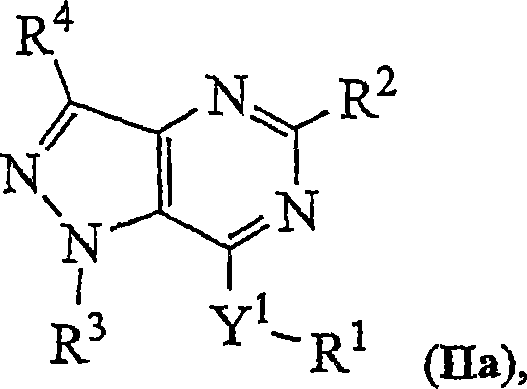
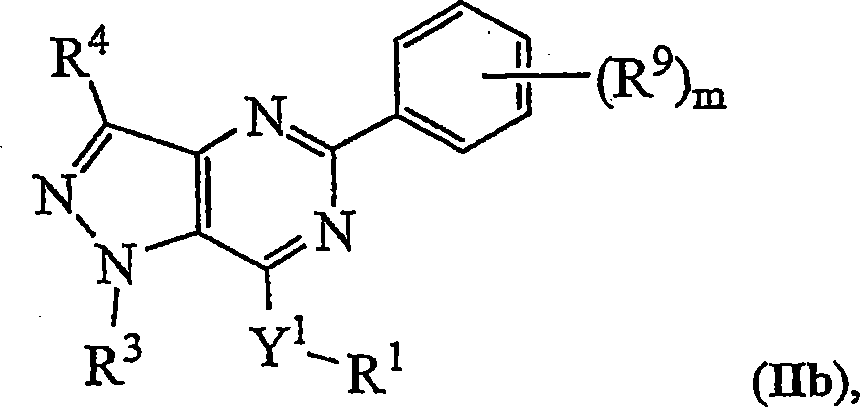
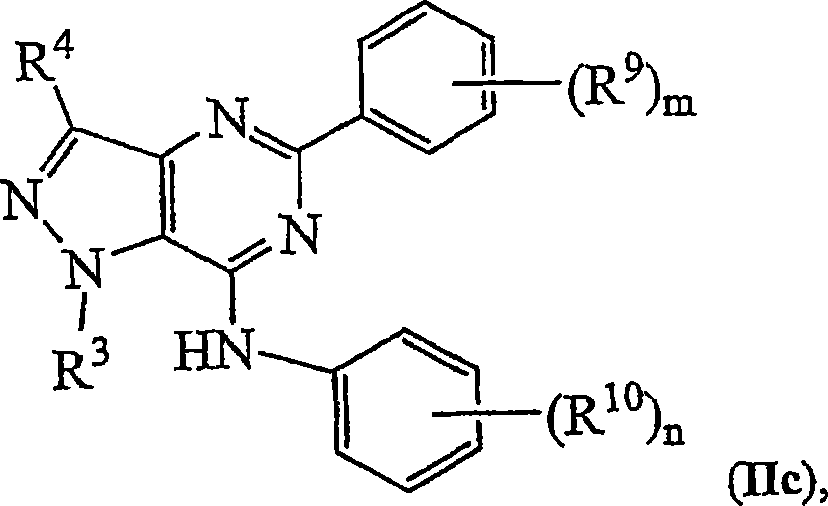
Novel bicyclic heterocyclic compounds, process for their preparation and compositions containing them
A compound and mixture technology, applied in the field of inflammation, or glycosidase expression-related conditions or diseases, in the field of treatment and cell proliferation, can solve the problems of ineffective curative effect and expensive treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0768]Preparation of (3-fluoro-4-methoxy-phenyl)-[5-(4-fluoro-phenyl)-1-methyl-3-propyl-1H-pyrazolo[4,3-d] Pyrimidin-7-yl]-amine hydrochloride (E1)
[0769]
[0770] Step 1: Preparation of 4-(4-fluoro-benzoylamino)-2-methyl-5-propyl-2H-pyrazole-3-carboxylic acid amide (3)
[0771]
[0772] a) Preparation of 4-fluorobenzoyl chloride (2). To a solution of 4-fluorobenzoic acid (10 g, 71.42 mmol) in dry ethyl acetate (EtOAc) (100 mL) was slowly added thionyl chloride (SOCl) at 10 °C under nitrogen atmosphere 2 ) (84.9 g, 714.2 mmol). The mixture was then stirred at 85°C for 12 hours. After the reaction is complete, the excess SOCl 2 Removal by low vacuum distillation afforded the desired compound, 4-fluorobenzoyl chloride (10.8 g, 95% yield). It was used directly in the next step without further purification.
[0773] b) Preparation of 4-(4-fluoro-benzamido)-2-methyl-5-propyl-2H-pyrazole-3-carboxylic acid amide (3). Add 4-amino-2-methyl-5-propyl-2H-pyrazole-3-carboxyl...
Embodiment 2-52
[0800] Unless otherwise indicated, the following compounds appearing in Examples 2-52 were prepared by methods similar to those disclosed in Example 1, using similar starting materials for appropriate substitutions, to obtain the corresponding compounds, listed as compounds E2-E52.
Embodiment 2
[0802] Preparation of (3-chloro-4-methoxy-phenyl)-[5-(4fluoro-phenyl)-1-methyl-3-propyl-1H-pyrazolo[4,3-d]pyrimidine -7-yl]-amine-hydrochloride (E2)
[0803]
[0804] Yield: 88%; Melting point: 253.65°C; 1 H NMR (200MHz, DMSO-d6 )δ 9.12(s, D 2 O exchangeable, 1H), 8.33-8.25(m, 2H), 7.92-7.91(m, 1H), 7.75-7.70(m, 1H), 7.35-7.23(m, 3H), 4.33(s, 3H) , 3.90(s, 3H), 2.92(t, J=7.3Hz, 2H), 1.82(q, J=7.3Hz, 2H), 0.97(t, J=7.5Hz, 3H); MS: 427(M + -35,100);IR(cm -1 ): 3441, 2949, 1626.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com