Application of recombination FN polypeptide in preparing medicine for treating severe infectious diseases such as septicemia
A technique for severe infection, sepsis, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
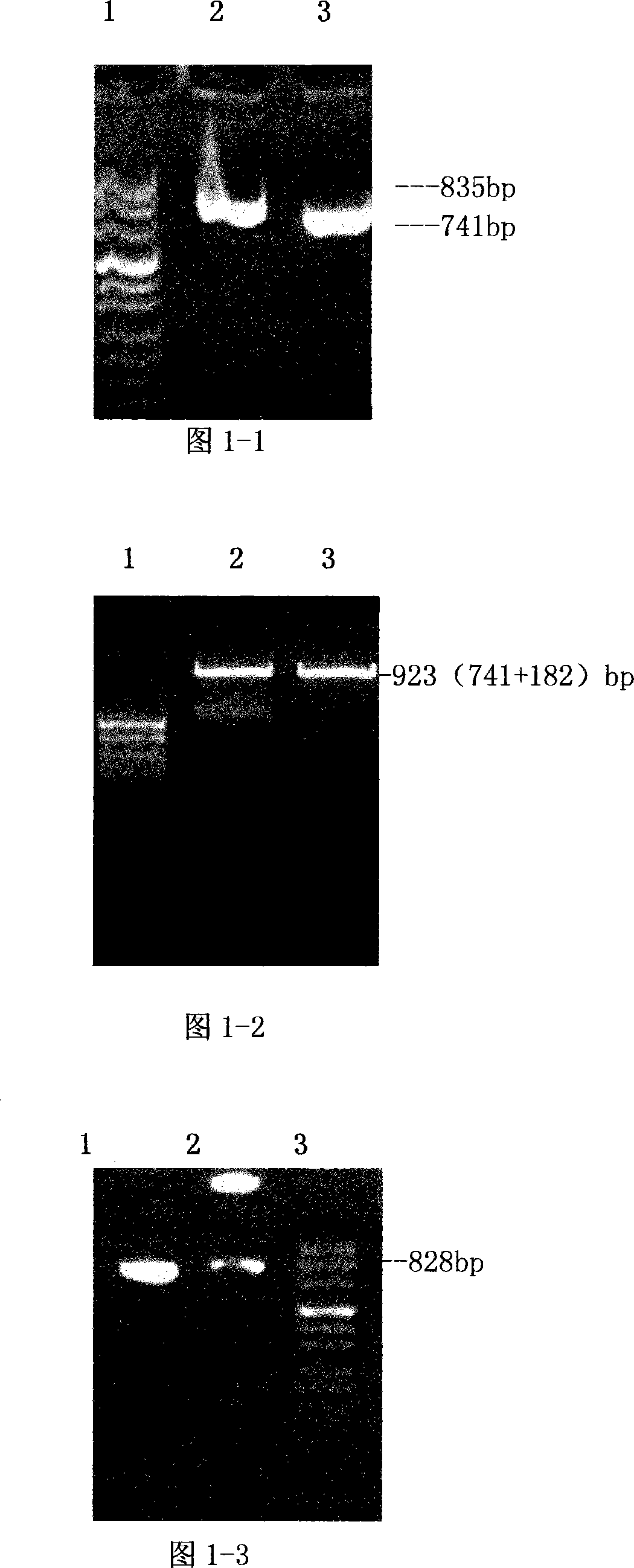
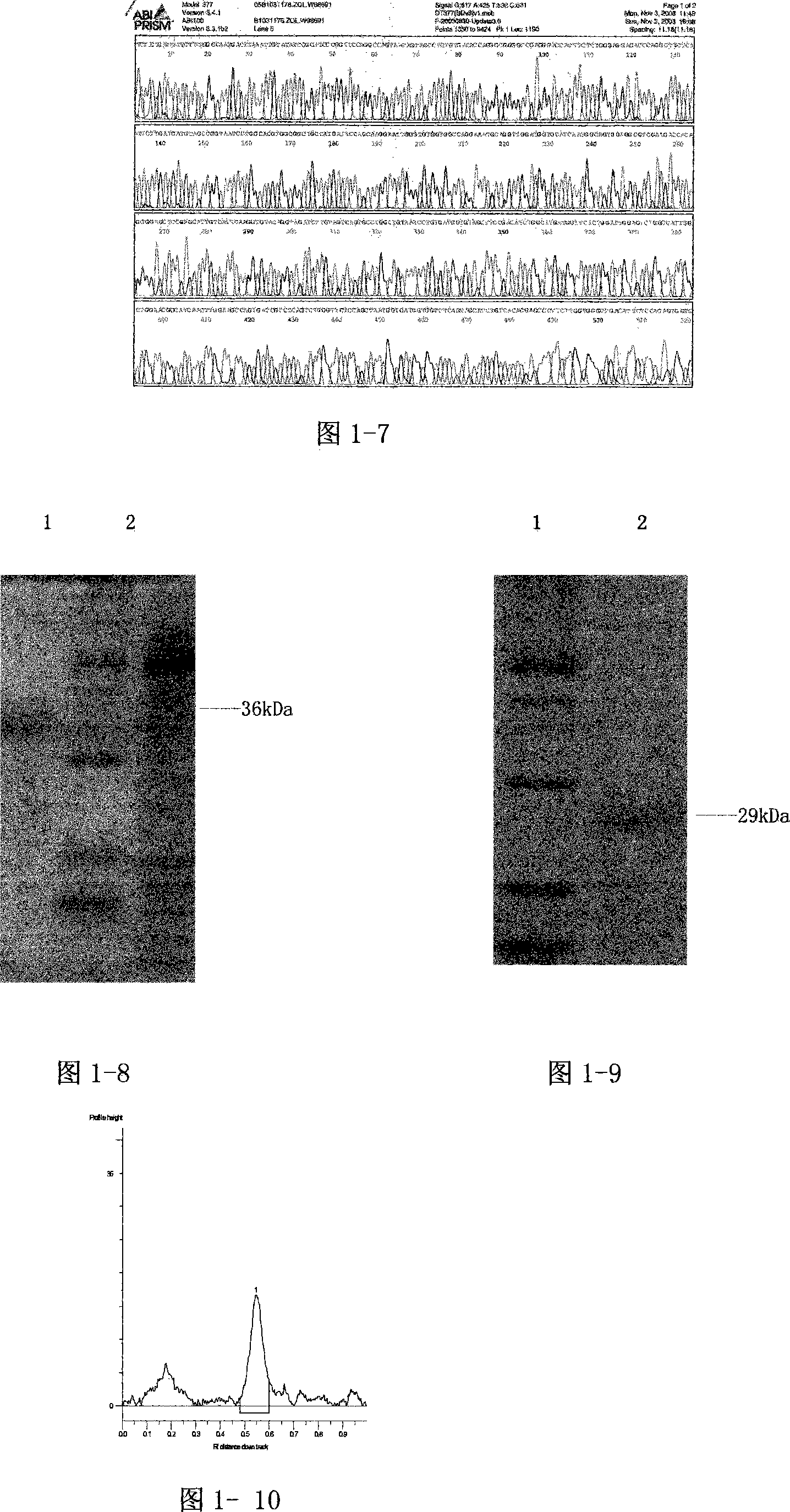
[0092] The present invention is described in detail below in conjunction with accompanying drawing and embodiment:
[0093] 1. Construction of recombinant FN heparin-binding domain polypeptide yeast expression vector and preparation of polypeptide
[0094] In the present invention, the heparin-binding domain polypeptide gene at the FNC end and the heparin-binding domain polypeptide gene at the FNN end are cloned into a yeast expression vector, and expressed in GS115 yeast cells.
[0095] 1.1) Design of primers for PCR amplification of the heparin-binding domain polypeptide gene at the FNC end and the FNN end
[0096] According to the FN cDNA sequence and the comparison with the amino acid sequence in the molecular structure of FN, the cloning positions of the fibronectin C-terminal heparin-binding domain polypeptide (rhFNHC-36) and the N-terminal heparin-binding domain polypeptide (rhFNHN-29) were determined, and yeast The enzyme cutting site of the expression vector, the PCR...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com