Vaccines based on the use of mv
一种痘苗病毒、病毒的技术,应用在病毒、抗病毒剂、病毒/噬菌体等方向,能够解决不足以实现治疗效果等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0089] Materials and methods
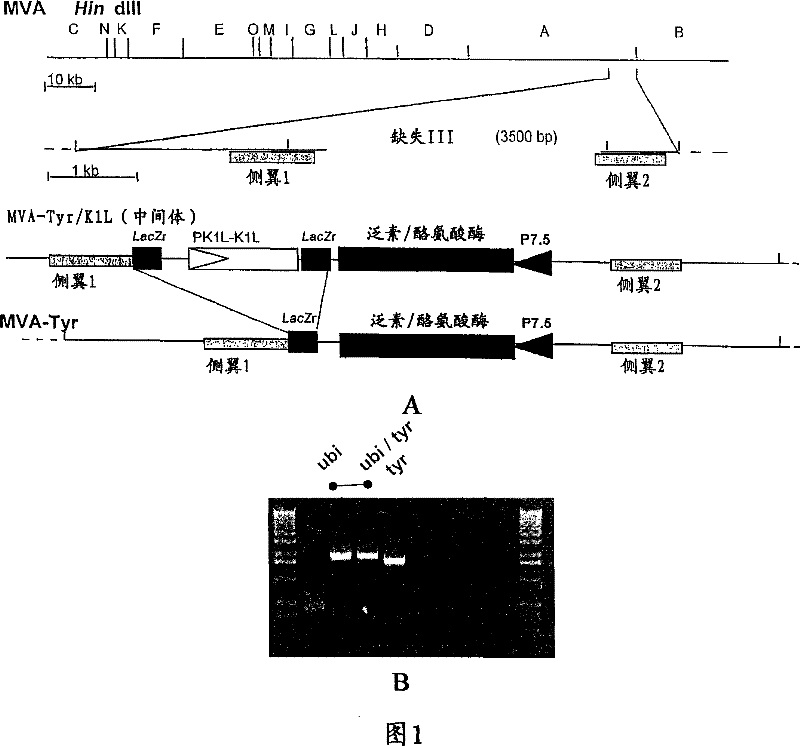
[0090] Plasmid construction
[0091] The ubiquitin / tyrosinase fusion gene was constructed and cloned into the MVA transfer vector pIIIdHR-P7.5. Here we establish a hybridization PCR method in which the ubiquitin (Ub) gene can be fused to the tyrosinase (hTyr) cDNA without insertion of any additional non-Ub or non-hTyr DNA sequences. Because ubiquitin expressed as a protein fusion is cleaved by cytoplasmic proteases at its G76 residue, we focused on mutating G76 to A76, which has been shown to inhibit cytoplasmic cleavage when expressed from a plasmid vector (Rodriguez F et al. J Virol, 1997).
[0092] In a first step, ubiquitin was amplified from RNA preparations of murine B16 melanoma cells in standard reverse transcriptase PCR according to the manufacturer's instructions (Titan One Tube RT-PCR System, Roche). Select primer 5′-GGG C GG ATC C GA CCA TGC AGA TCT TCGTGA AGA CCC TGAC-3′ and 5′-CAA AAC AGC CAG GAG CAT CGC ACCTCT CAG GCG A...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com