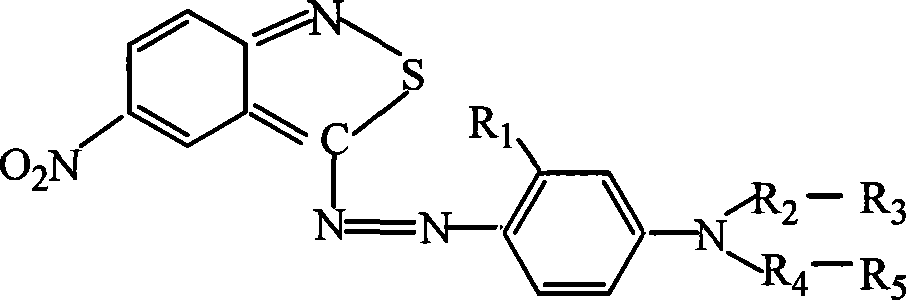
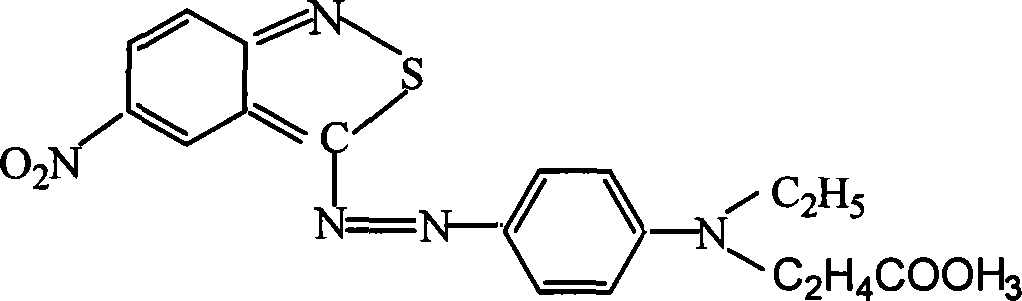
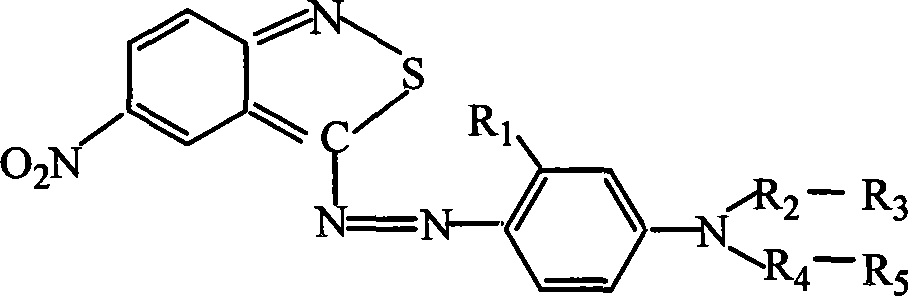
Azo type heterocyclic blue dispersion dyes
A technology of disperse dyes and heterocycles, used in azo dyes, monoazo dyes, organic dyes, etc., can solve the problems of poor dyeing performance, sensitivity to pH value of dyeing bath, etc., achieve excellent fastness and dyeing performance, environmental protection The effect of good sex and high color power
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Dissolve 19.5 grams of 3-amino-5-nitro-2,1-benzoisothiazole (0.1 mole) in 50 milliliters of sulfuric acid and stir to dissolve, control at 10-15 ° C, dropwise add 32.4 grams of 40% nitrous Acyl sulfuric acid (0.102 mol) was kept at 10-15°C for 6 hours to obtain diazonium solution. At the same time, 24.6 grams of N-isopropyl N-(β-cyanoethoxyethyl)-m-toluidine (0.1 mol) was dissolved in 10 ml of sulfuric acid and 300 ml of water to form a coupling component, and an appropriate amount of crushed ice was added. Drop below 0°C, then slowly drop the diazo solution into the coupling solution, control the reaction at 0-5°C until the diazo component disappears, filter, wash with water until neutral, exhaust, and dry to obtain dye 36-38 grams, dyed polyester fiber is brilliant blue.
Embodiment 2
[0019] The dry product dye obtained in Example 1 is mixed with the dispersant in a ratio of: dye (dry product): dispersant MF: sodium lignosulfonate: water=1: 1: 1: 6.5 sand milled to a particle size of less than 1U , dried to obtain commercial dyes. Add 0.1 gram of the above-mentioned commercial dye to a dye vat containing 200 ml of water, add 5 grams of polyester fabric after the dye is completely dissolved, seal the dye vat and put it into the dyeing machine, slowly raise the temperature, rise to 130°C for about 30 minutes and keep it warm for 45 minutes. After cooling down, the dyed sample was taken out, and it was bright blue after soaping.
Embodiment 3~22
[0021] In each embodiment, the amount of 3-amino-5-nitro-2,1-benzoisothiazole is 0.1 mole, and the ratio (mole) and operation of other materials are the same as in Example 1 to obtain a series of heterocyclic Blue disperse dye. The structure of each coupling component, see below
[0022] surface:
[0023] Example
[0024] Example
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com