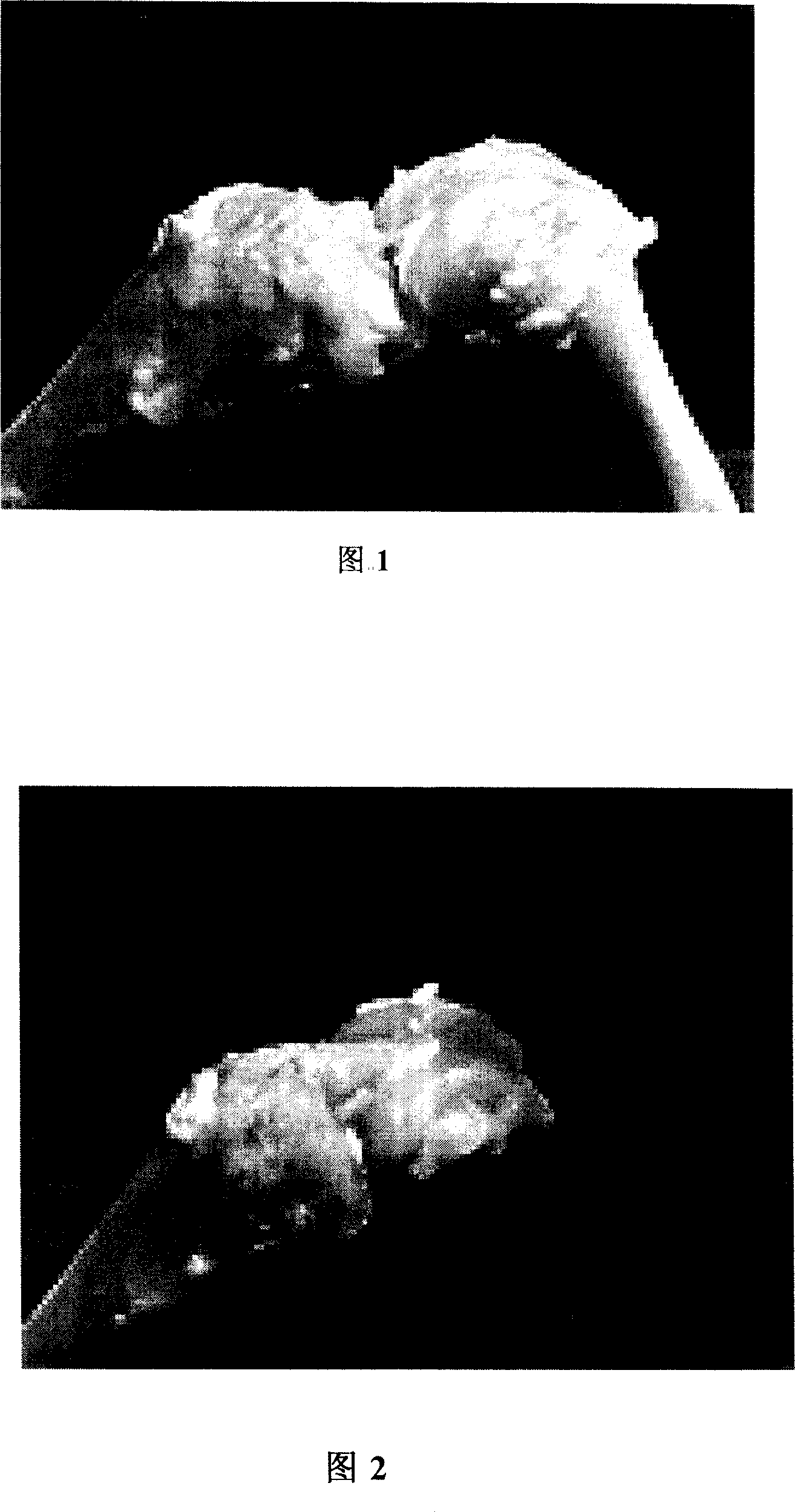
Composition for treating knee-joint cross ligament damage
An anterior cruciate ligament and composition technology, which is applied in the field of therapeutic pharmaceutical compositions for knee anterior cruciate ligament injury, can solve problems such as no cure for anterior cruciate ligament, and achieve the effect of significant curative effect and inhibition of excessive release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1: Composition formula 1
[0027] Composition ingredients: retinoic acid 50ng / ml, tetracycline 10 -4 M, curcumin 50μM, transforming growth factor 10ng / ml.
[0028] Preparation method:
[0029] Dilute with 2.5 mL of chilled PBS, adjust the pH to 7.4 with 0.2 M NaOH, and maintain at 250-300 milliosmolar (mOsm). The composition was added to the pH-adjusted solution to achieve a final total concentration of 2 mg / mL.
[0030] Experimental animals: Wistar rats weighing 220±20 g, male, in three groups, 10 in each group.
[0031] The two groups of rats underwent ACL rupture surgery, and then they were reunited.
[0032] After one group was reunited, in order to alleviate the postoperative pain of the rats, in the first 5 days, 0.01-0.05mg / ml of analgesics was injected every 12 hours to allow them to recover automatically.
[0033] In the other group, 3 μL of sterilized collagen gel was applied to the ACL-ACL suture after the surgery. After 50 minutes, 5 μL of the...
Embodiment 2
[0037] Embodiment 2: Composition formula 2
[0038] Ingredients: Tretinoin 50ng / ml, Tetracycline 10 -4 M, curcumin 50μM, transforming growth factor 30ng / ml.
[0039] Preparation method: with embodiment 1
[0040] Experimental animals: Wistar rats weighing 220±20 g, male, in three groups.
[0041] Test process: with embodiment 1
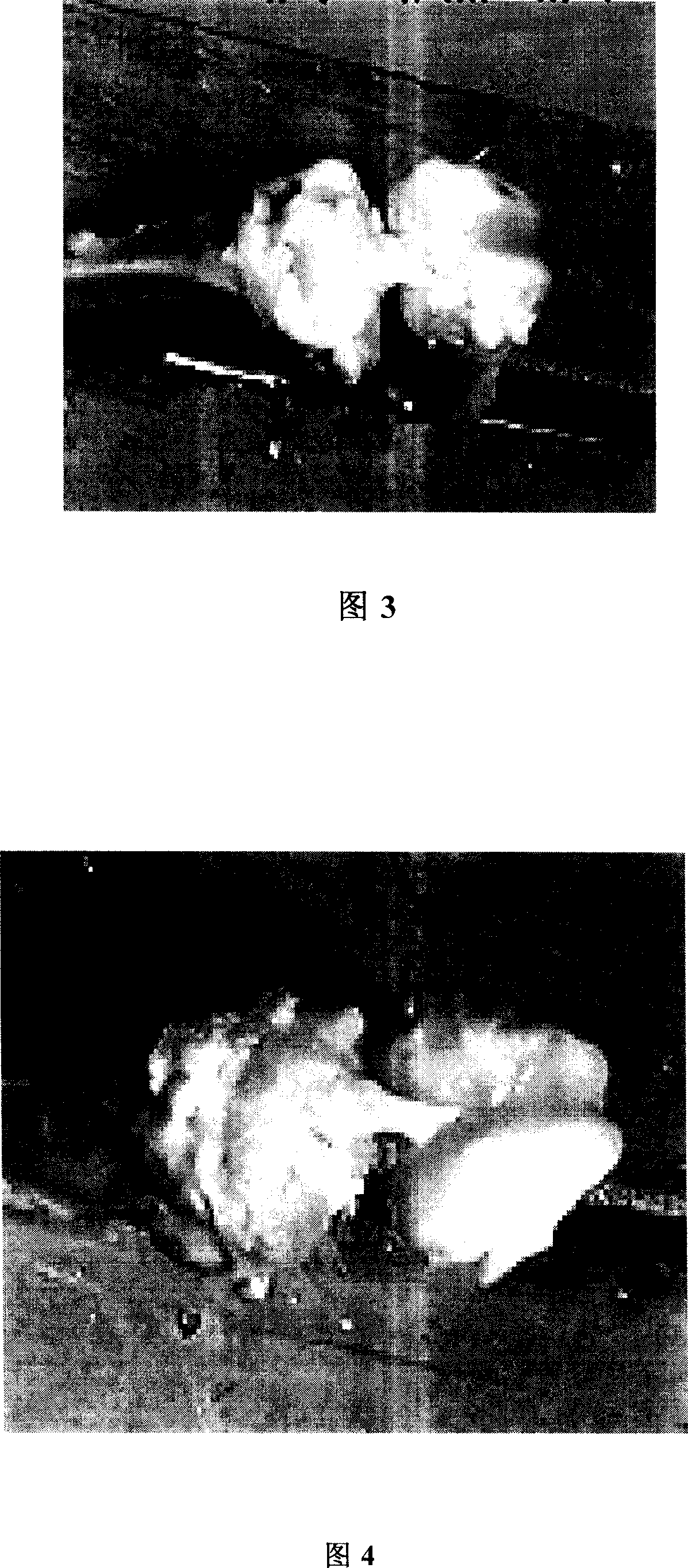
[0042] 6 weeks later. All the rats were sacrificed, and the bone-ligament-bone complex was taken out, see Fig. 3, and the tensile strength of the ligament was measured. Result table two:
[0043] Table II
[0044] group
Embodiment 3
[0045] Embodiment 3: Composition formula 3
[0046] Composition ingredients: retinoic acid 50ng / ml, tetracycline 10 -4 M, NF-κB inhibitor 50μM, transforming growth factor 30ng / ml.
[0047] Preparation method: with embodiment 1
[0048] Experimental animals: Wistar rats weighing 220±20 g, male, in three groups.
[0049] Test process: with embodiment 1
[0050] 6 weeks later. All the rats were sacrificed, and the bone-ligament-bone complex was taken out, see Fig. 4, and the tensile strength of the ligament was measured. Result table three:
[0051] Table three
[0052] group
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com