Human resource vessel endothelium growth factor monoclonal antibody and preparation method thereof
A monoclonal antibody and vascular endothelial technology, applied in the fields of genetic engineering and immunology, can solve problems such as poor stability, low activity, and low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
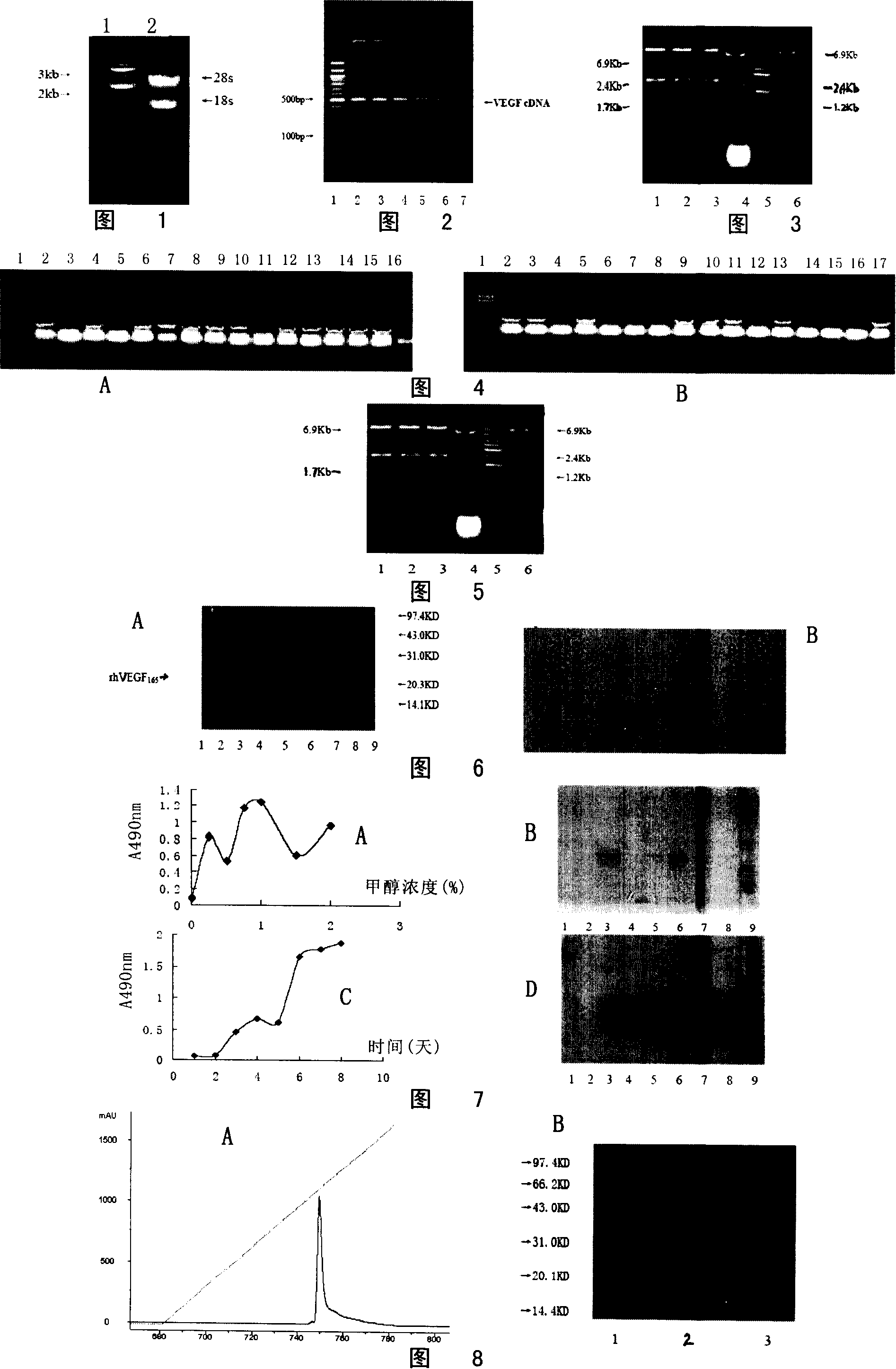
[0085] Embodiment 1 human vascular endothelial growth factor (VEGF 165) preparation
[0086] 1. Human vascular endothelial growth factor (VEGF 165 ) cDNA cloning and identification
[0087] 1. Extraction of human tumor total RNA
[0088] One tumor-bearing nude mouse was sacrificed, and the tumor tissue was removed aseptically. After homogenization in an ice bath, 1ml TriZol was added to 100mg of tissue. Place on ice for about 5 minutes until the tissue is completely lysed. An equal volume of phenol:chloroform (1:1) was added, and vortexed for 1 min. Place on ice for 5min, centrifuge at 12000rpm, 4°C for 10min. Carefully absorb the supernatant, add an equal volume of chloroform, mix well, place on ice for a while, and centrifuge at 12,000 rpm at 4°C for 5 min. Aspirate the supernatant, add twice the volume of ethanol, mix well, and place at -20°C for 30min. 2000rpm, 4°C, centrifuge for 10min. Drain the supernatant, wash with 70% alcohol, let it dry until there is no al...
Embodiment 2
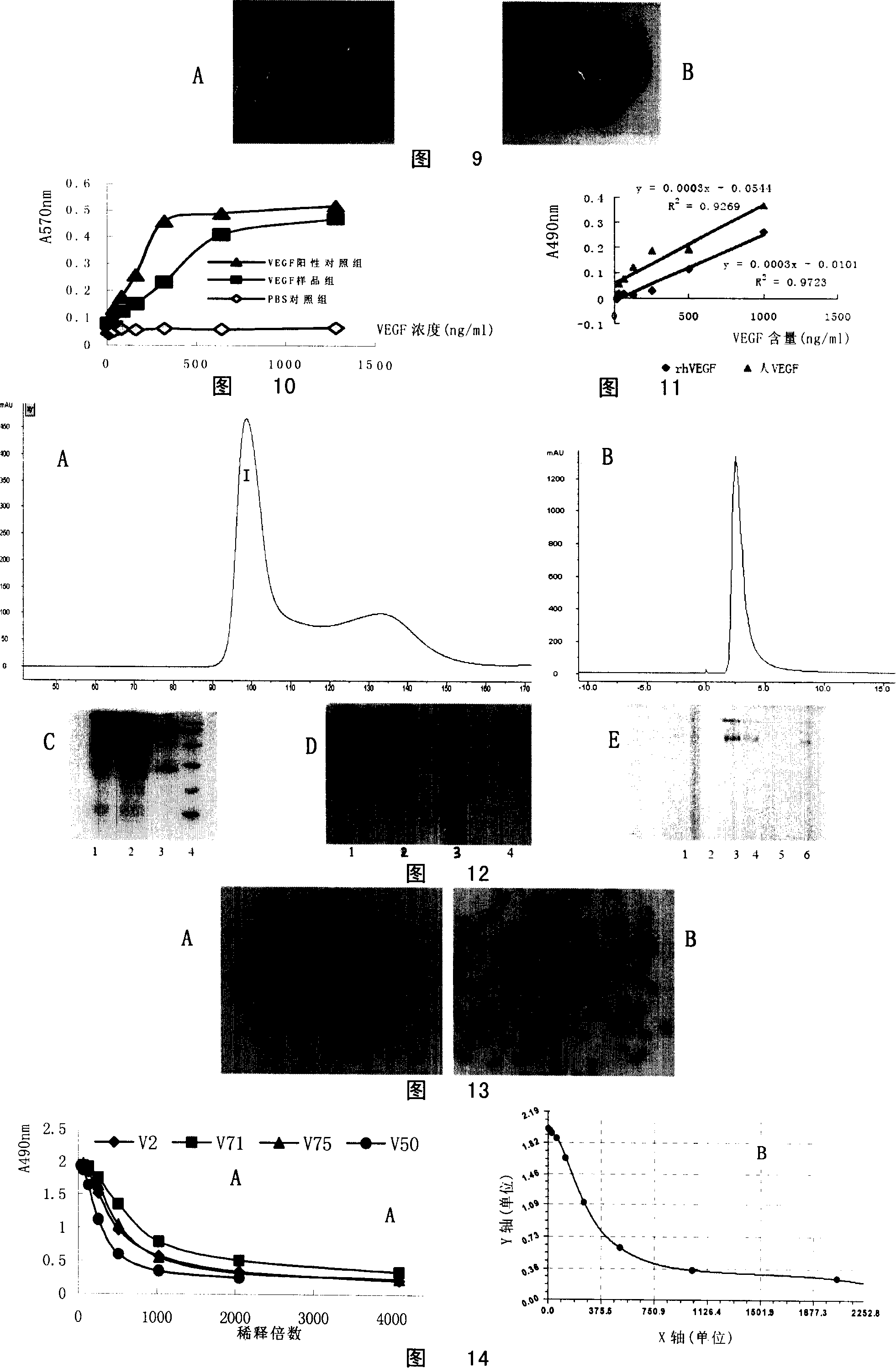
[0145] Example 2 Preparation, purification and characteristic research of human anti-VEGF monoclonal antibody
[0146] 1. Preparation of human anti-VEGF monoclonal antibody
[0147] 1. Animal immunization: adopt subcutaneous and intraperitoneal two immunization routes to immunize five-characteristic mice (purchased from Babraham Institute, UK), and the specific methods are as follows:
[0148] (1) Take an appropriate amount of the purified VEGF sample prepared in Example 1, dilute it with PBS to the desired concentration, mix it with immune adjuvant at a ratio of 1:1, and fully emulsify it for later use. Complete Freund's adjuvant (CFA) was used for initial immunization, and incomplete Freund's adjuvant (IFA) was used for booster immunization.
[0149] (2) Subcutaneous immunization method: inject 0.01ml subcutaneously on the sole of one foot, and inject the remaining part subcutaneously on both sides of the neck.
[0150] (3) Intraperitoneal injection method: intraperitoneal...
Embodiment 3
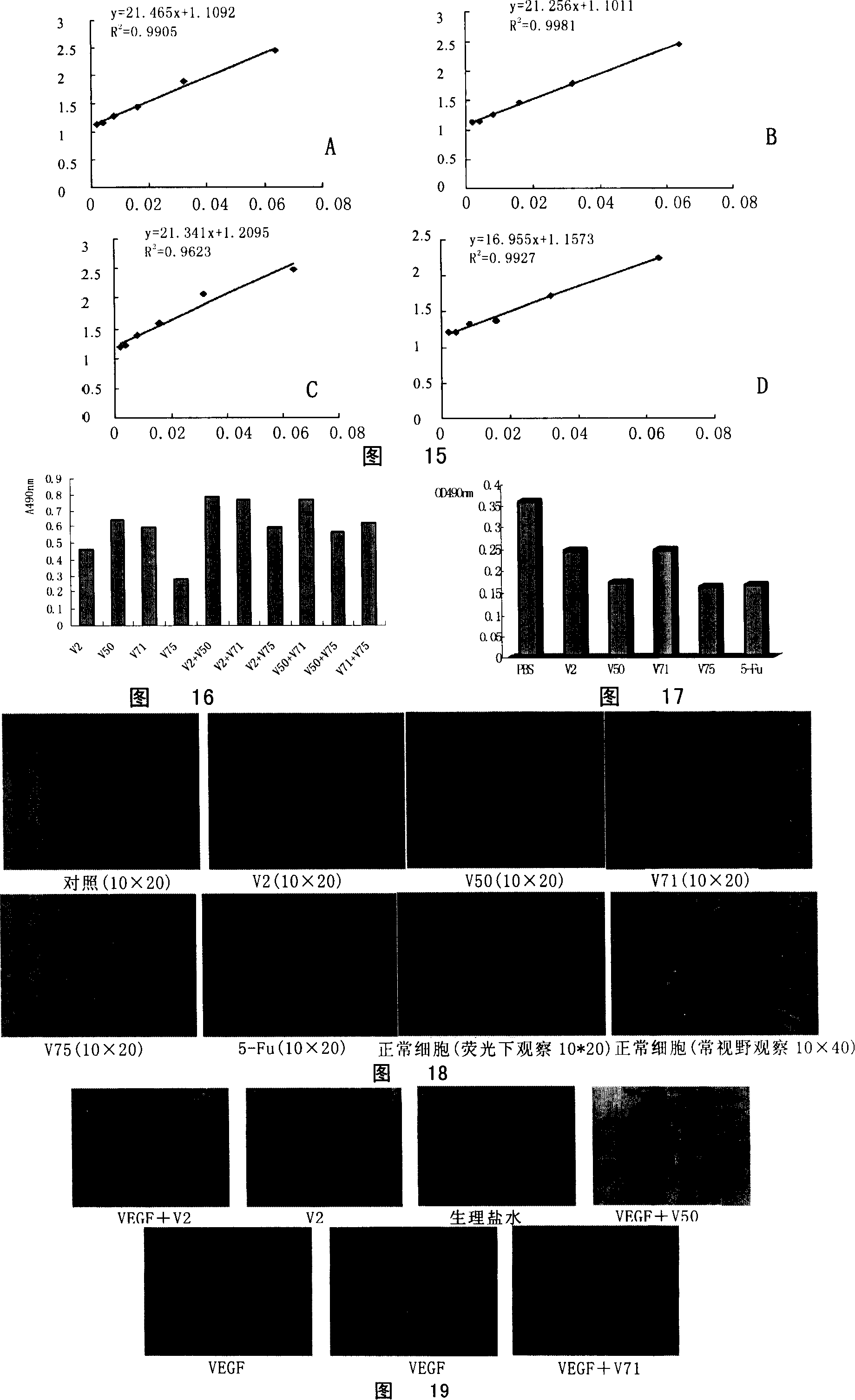
[0211] Example 3 Activity Research of Human Anti-VEGF Monoclonal Antibody
[0212] 1. In vitro activity research
[0213] 1. ELISA method to measure the resistance effect of human monoclonal antibody on the binding of VEGF to its receptor VEGFR2
[0214] Method 1: After coating the plate with VEGFR2 (purchased from sigma), add 1 nM VEGF and different concentrations of enzyme-labeled human antibody (HRP-labeled) to incubate for 1 hour, develop color, and measure the light absorbance at 490 nm.
[0215] Method 2: After wrapping the plate with VEGFR2, add 1nM VEGF, incubate at 37°C for 1hr, add different concentrations of enzyme-labeled human antibody (HRP-labeled), incubate at 37°C for 1hr, develop color, and measure the light absorption at 490nm value.
[0216] The results are shown in Table 6.
[0217] Table 6 Impedance effect of human monoclonal antibody on the binding of VEGF to its receptor VEGFR2
[0218] V2
V50
V71
V75
Law one
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com