Function of 2',2'-CH3-2'H,4H-3,6'-two benzopyran-4-ketone
A compound and pharmaceutical technology, applied in the field of medicine, can solve the problems such as unclear role of non-classical estrogen membrane signaling pathway
Active Publication Date: 2008-06-18
BEIJING SHENOGEN BIOMEDICAL
View PDF0 Cites 2 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
However, the role of the non-canonical estrogen membrane signaling pathway is still unclear
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
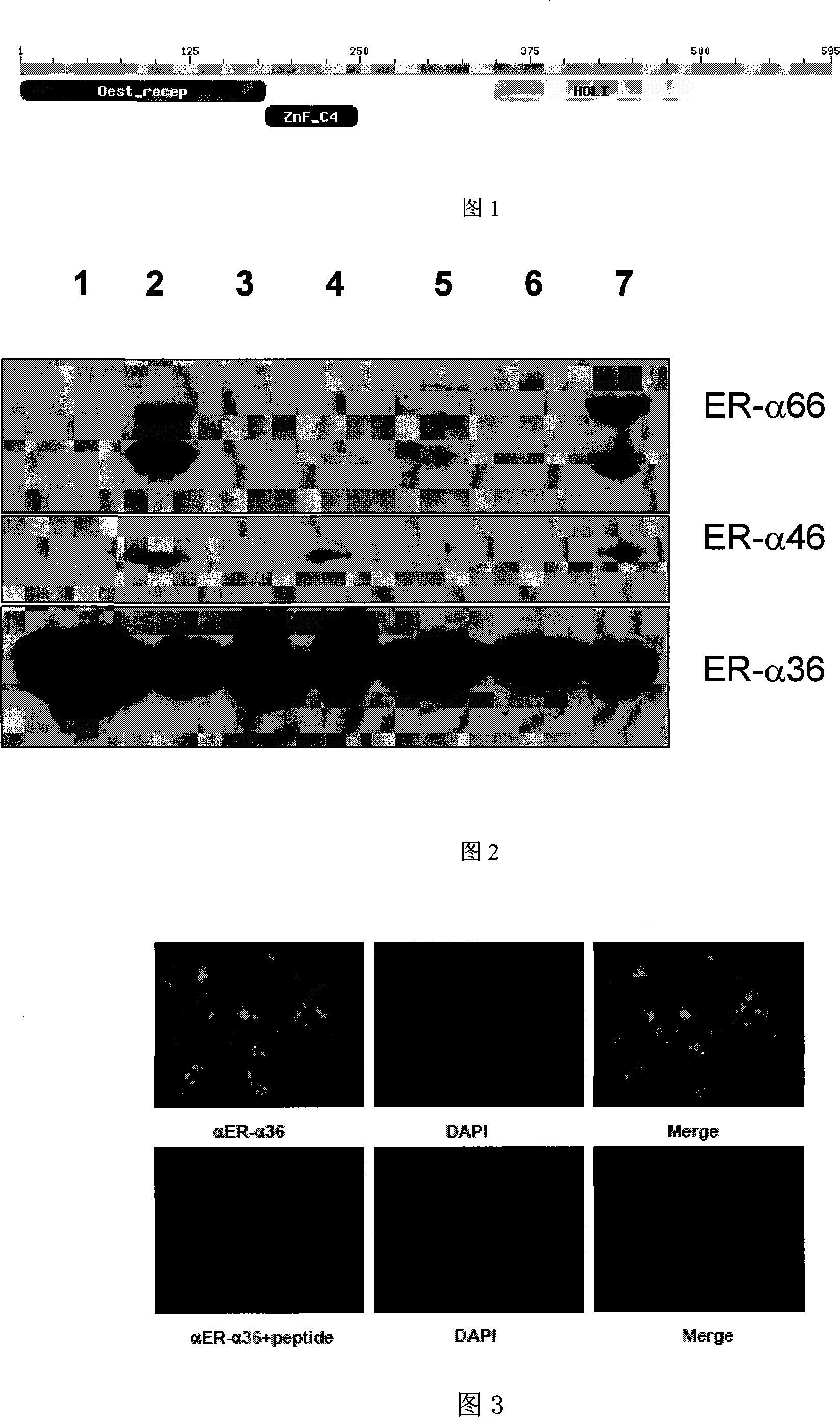
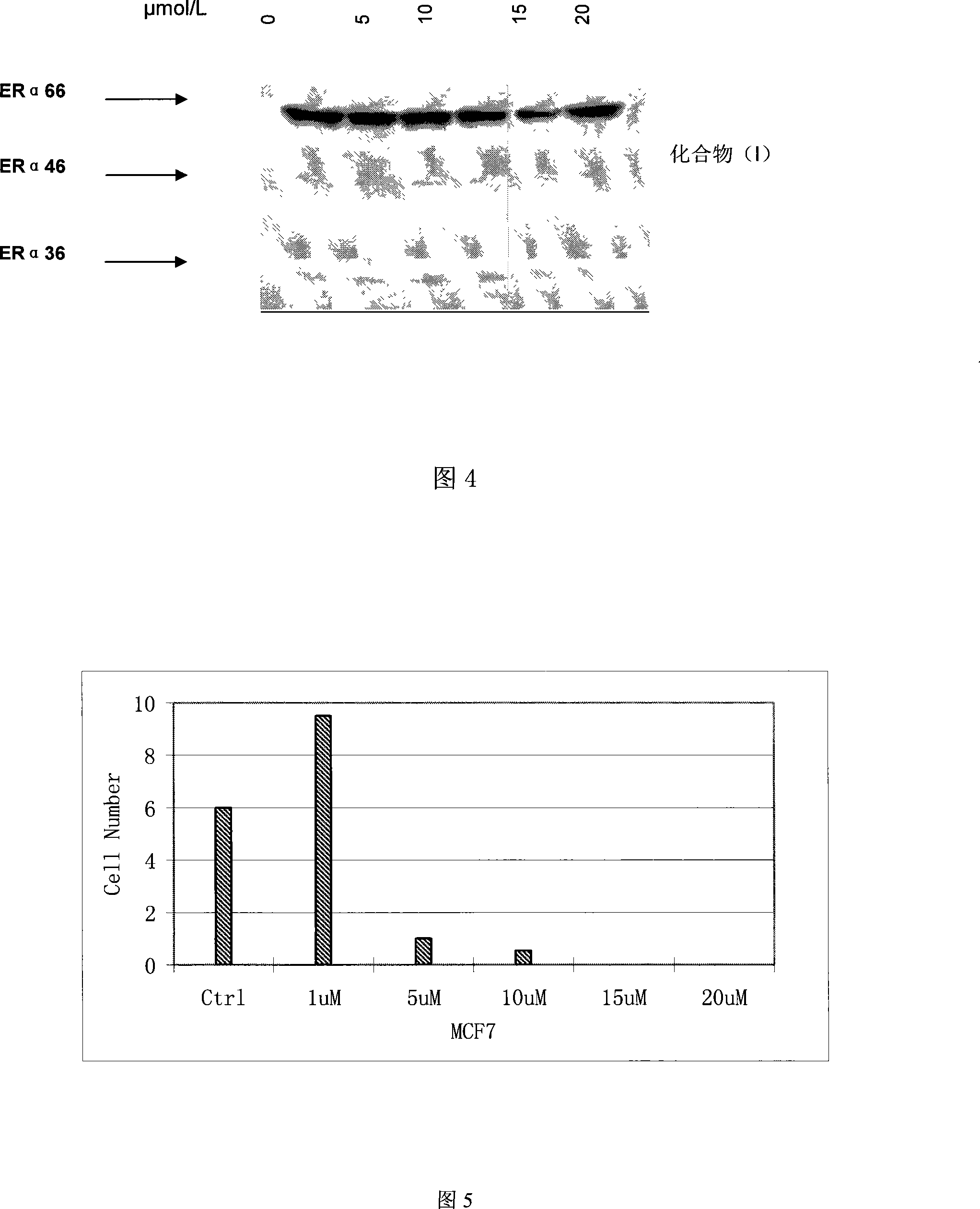
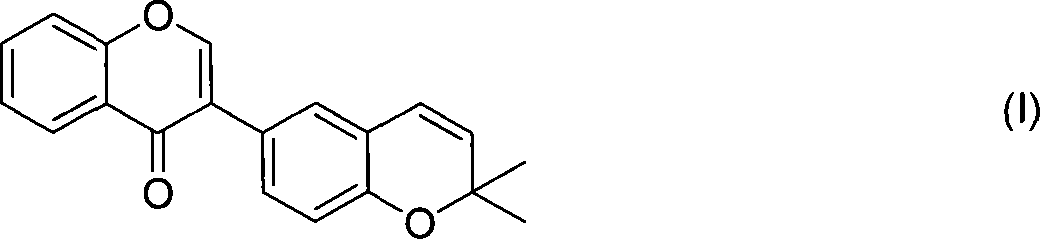
The invention relates to an application of 2`,2`-dimethyl-2`H, 4H-3, 6`-bis- benzopyran-4-ketone , in particular to an application of the compound 2`,2`-dimethyl-2`H, 4H-3, 6`-bis- benzopyran-4-ketone in preparing drugs for treating related diseases such as estrogen receptor ER-Alpha subtype and ER-Beta subtype, belonging to the medicine field. In the condition of low concentration, the compound does not change the expressions of ER-Alpha66, ER-Alpha46 and ER-Alpha36; while in the condition of high concentration, the compound can inhibit the expressions of the three at the same time. Meanwhile, the compound can kill breast cancer cell MCF7 that expresses the ER-Alpha66, the ER-Alpha46 and the ER-Alpha36. Therefore, the compound can be also used as the regulator of the estrogen receptor ER-Alpha36 to treat diseases caused by abnormal expression of the estrogen receptor ER-Alpha36, such as tumor, osteoporosis, asthma, heart disease or senile dementia , etc.
Description
technical field [0001] The invention belongs to the field of medicine and relates to the application of natural compounds in the preparation of antitumor drugs, osteoporosis and senile dementia drugs. Background technique [0002] Estrogen is a steroid hormone produced by the endocrine system, which plays an important role in the reproductive system, bone tissue, cardiovascular system, immune system and central nervous system [J Cell Sci, 2003; 116(4): 585-586]. The estrogen signaling system plays an important role in the regulation of cell growth, differentiation and apoptosis. The occurrence and development of estrogen-dependent tumors, such as breast cancer, ovarian cancer and endometrial cancer, are closely related to estrogen [J Steroid Biochem.Mol.Biol., 2002, 81(1): 1-24 , J Mammary Gland Biol. Neoplasia, 1998, 3(1): 49-61, Curr. Drug Targets Immune Endocr. Metabol. Disord; 2001, 1(1): 1-12, Cancer Res., 1998, 58(23) : 5367-5373, J Psychiatry Neurosci, 2002; 27 (1)...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): A61K31/352A61K9/08A61K9/10A61K9/20A61P35/00
Inventor 李靖孟坤
Owner BEIJING SHENOGEN BIOMEDICAL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com