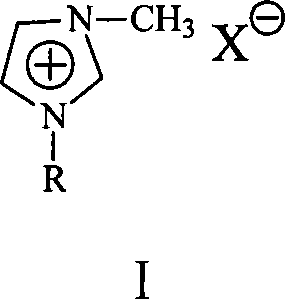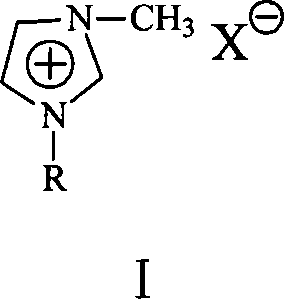Process for producing cyclohexylbenzene
A technology for cyclohexylbenzene and cyclohexene is applied in the field of preparation of cyclohexylbenzene, and can solve the problems of high corrosiveness of catalysts, decreased activity and rate of alkylation reactions, expensive alkylation reagents, and the like.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] In a 500ml jacketed reactor, a super constant temperature tank is used for heating in a water bath, and an electric stirring paddle is used for stirring. The rotation speed is greater than 500 rpm, which is enough to eliminate mass transfer and heat transfer resistance. In the reactor, add 262.2 grams of benzene, 18.4 grams of cyclohexene, 15 grams of Et 3 NHCl-ZnCl 2 Ionic liquid, react at 60°C. After 30 minutes of reaction, the conversion of cyclohexene was 100%. After the catalyst was separated from the product, atmospheric distillation was carried out to remove excess benzene, and vacuum distillation was carried out to obtain 34.0 g of colorless liquid fractions with a product yield of 94.9%. The product was analyzed by GC-MS, and the purity of the cyclohexylbenzene product was found to be 99.8%.
[0025] The peaks of 104 and 160 appear in the MS spectrum, which are respectively knocked out (-CH 2 ) 4 The final fragment peak and the molecular ion peak of cycloh...
Embodiment 2
[0027] The basic steps are the same as in Example 1, and the catalyst is 15 grams of [bmin]Br-AlCl 3 Ionic liquid ([bmin]Br is 1-butyl-3-methylimidazolium bromide salt, the same below). After 30 minutes of reaction, the conversion of cyclohexene was 100%. After the catalyst was separated from the product, atmospheric distillation was carried out to remove excess benzene, and vacuum distillation was carried out to obtain 34.2 g of colorless liquid fractions with a product yield of 95.4%. The product is analyzed by GC-MS, and the purity of the cyclohexylbenzene product is 99.9%.
Embodiment 3
[0029] The basic steps are the same as in Example 1, and the temperature is 40°C. After 60 minutes of reaction, the conversion of cyclohexene was 99.8%. After the catalyst was separated from the product, atmospheric distillation was carried out to remove excess benzene, and vacuum distillation was carried out to obtain 33.8 g of colorless liquid fractions with a product yield of 94.3%. The product is analyzed by GC-MS, and the purity of the cyclohexylbenzene product is 99.85%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com