3-substituted oxygen group-3',4'-dimethoxy flavonoid compound with blood fat reducing function
A technology of dimethoxyflavone and compound, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
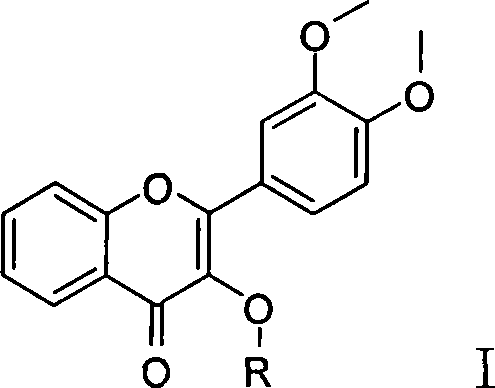
[0042] Example 1 Preparation of 3-acetoxy-3', 4'-dimethoxyflavone (compound 2) (R=acetyl in the general formula I)
[0043] Take 100mg of compound 1, 10ml of acetic anhydride, 1 drop of concentrated sulfuric acid, and stir at room temperature for 3h. Then, 100 ml of water was added to the reaction liquid, and stirring was continued overnight. After filtration, the solid was dried and recrystallized with isopropanol to obtain compound 2 as a slightly yellow solid with a melting point of 106-108° C. and a yield of 70%.
[0044] 1 HNMR (CDCl 3 )δ, ppm: 2.37 (3H, s, -CH 3 ), 3.95, 3.97 (6H, d, -OCH 3), 6.98~7.01(1H, d, 2'-H), 7.41~7.43(2H, m, 5', 6'-H), 7.52~7.58(2H, m, 6, 8-H), 7.69~ 7.74(1H, m, 7-H), 8.25~8.26(1H, m, 5-H).
[0045] MS(ESI)M + =341
Embodiment 2
[0046] Example 2 Preparation of 3-propionyloxy-3', 4'-dimethoxyflavone (compound 3) (R = propionyl in general formula I)
[0047] Take 100 mg of compound 1 and 10 ml of propionic anhydride, and follow the method of Example 1 to obtain compound 3 as a light yellow solid with a melting point of 148-150° C. and a yield of 60%.
[0048] 1 HNMR (CDCl 3 )δ, ppm: 1.26 ~ 1.30 (3H, t, -CH 3 ), 2.68~2.70 (2H, m, -CH 2 -), 3.96, 3.977 (6H, d, -OCH 3 ), 6.98~7.01(1H, d, 2'-H), 7.42~7.47(2H, m, 5', 6'-H), 7.52~7.58(2H, m, 6, 8-H), 7.69~ 7.72(1H, m, 7-H), 8.25~8.27(1H, m, 5-H).
[0049] MS(ESI)M + =355
Embodiment 3
[0050] Example 3 Preparation of 3-butyryloxy-3',4'-dimethoxyflavone (compound 4) (R=butyryl in general formula I)
[0051] Take 100mg of compound 1, 65mg of NaOH, 5ml of water, and 5ml of THF. Add 5ml of THF solution containing 1ml of butyryl chloride at a temperature below 5°C. After dropping, continue to react at this temperature for 1h, then pour the reaction solution into 50ml of water, and filter the precipitated solid. Recrystallized from isopropanol to obtain compound 4 as a pale yellow solid with a melting point of 113-116°C.
[0052] 1 HNMR (CDCl 3 )δ, ppm: 1.01 ~ 1.06 (3H, t, -CH 3 ), 1.77~1.84 (2H, m, -CH2-), 2.60~2.65 (2H, t, -OCH 2 -), 3.96~3.99 (6H, d, -OCH 3 )6.98~7.01(1H, d, 2'-H), 7.41~7.44(2H, m, 5', 6'-H), 7.52~7.58(2H, m, 6, 8-H), 7.69~7.75 (1H, m, 7-H), 8.25~8.28 (1H, m, 5-H).
[0053] MS(ESI)M + =369
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com