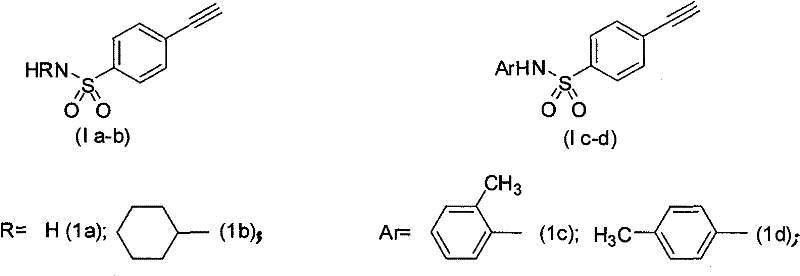
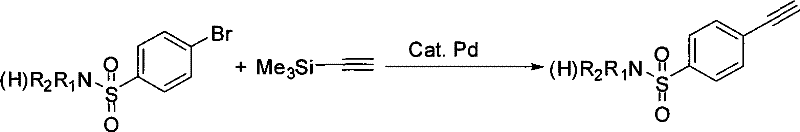
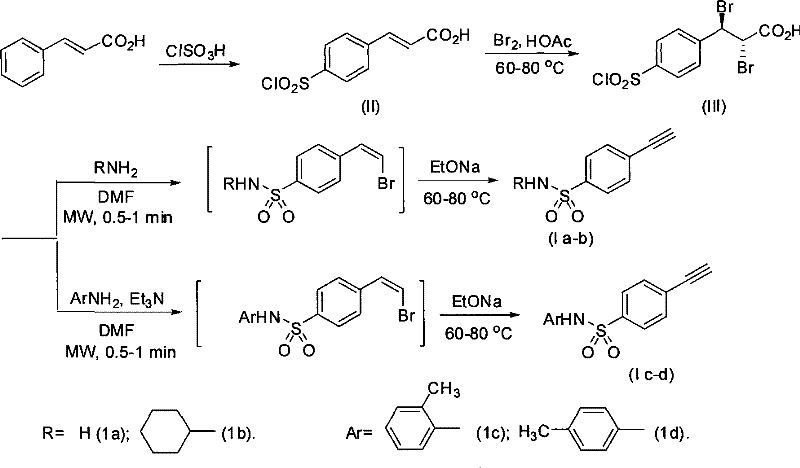
Process for producing 4-ethynyl benzene sulfonamide (I)
A technology of ethynylbenzenesulfonamide and chlorosulfonylphenyl, which is applied in the field of preparation of 4-ethynylbenzenesulfonamide, can solve the problems of two kinds of raw materials and metal catalysts being expensive and restricting large-scale production, and achieves simple and convenient operation, Reduction of purification steps and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] (1) Preparation of trans-4-chlorosulfonyl cinnamic acid (II)
[0031] resolve resolution:
[0032]
[0033] Chlorosulfonic acid (60 mL, 0.9 mol) was placed in a 250 mL round bottom flask and cooled to 0 °C with an ice bath. Cinnamic acid (14.8 g, 0.1 mol) was added to the flask in 8 portions over 1.5 hours. Control the reaction temperature at 0°C for 18 hours. The ice bath was removed, and the reaction solution naturally returned to room temperature. After heating to 60° C. for 0.8 hours, the oil bath was removed again, and the reaction solution was naturally cooled to room temperature. The resulting brown-red reaction solution was slowly poured into 600 mL of ice water. Let stand, filter, wash the filter cake with ice water, recrystallize in 300mL glacial acetic acid, filter, and the filtrate is a white solid. P 2 o 5 After drying, white trans-4-chlorosulfonyl cinnamic acid (II) is obtained. Wherein, the molar ratio of chlorosulfonic acid and cinnamic acid is...
Embodiment 2
[0042] (1) Preparation of trans-4-chlorosulfonyl cinnamic acid (II)
[0043] Chlorosulfonic acid (80 mL, 1.2 mol) was placed in a 250 mL round bottom flask and cooled to 0 °C with an ice bath. Cinnamic acid (14.8 g, 0.1 mol) was added to the flask in 10 portions over 2.5 hours. Control the reaction temperature at 0°C for 16 hours. The ice bath was removed, and the reaction solution naturally returned to room temperature. After being heated to 55° C. for 1.2 hours, the oil bath was removed again, and the reaction solution was naturally cooled to room temperature. The resulting brown-red reaction solution was slowly poured into 600 mL of ice water. Let stand, filter, wash the filter cake with ice water, recrystallize in 300mL glacial acetic acid, filter, and the filtrate is a white solid. P 2 o 5 After drying, white trans-4-chlorosulfonyl cinnamic acid was obtained. Wherein, the molar ratio of chlorosulfonic acid and cinnamic acid is 12:1.
[0044] (2) Preparation of 3-(4...
Embodiment 3
[0051] (1) Preparation of trans-4-chlorosulfonyl cinnamic acid (II)
[0052] Chlorosulfonic acid (60 mL, 0.9 mol) was placed in a 250 mL round bottom flask and cooled to 0 °C with an ice bath. Cinnamic acid (14.8 g, 0.1 mol) was added to the flask in 8 portions over 1.5 hours. Control the reaction temperature at 0°C for 18 hours. The ice bath was removed, and the reaction solution naturally returned to room temperature. After heating to 60° C. for 0.8 hours, the oil bath was removed again, and the reaction solution was naturally cooled to room temperature. The resulting brown-red reaction solution was slowly poured into 600 mL of ice water. Let stand, filter, wash the filter cake with ice water, recrystallize in 300mL glacial acetic acid, filter, and the filtrate is a white solid. P 2 o 5 After drying, white trans-4-chlorosulfonyl cinnamic acid was obtained. Wherein, the molar ratio of chlorosulfonic acid and cinnamic acid is 9:1.
[0053] (2) Preparation of 3-(4-chloro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com