Chloride 1,3-di(2-hydroxy ethyl) imidazole ionic liquid and method for synthesizing same
A technology of imidazolium ionic liquid and hydroxyethyl, applied in the field of chloride 1
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
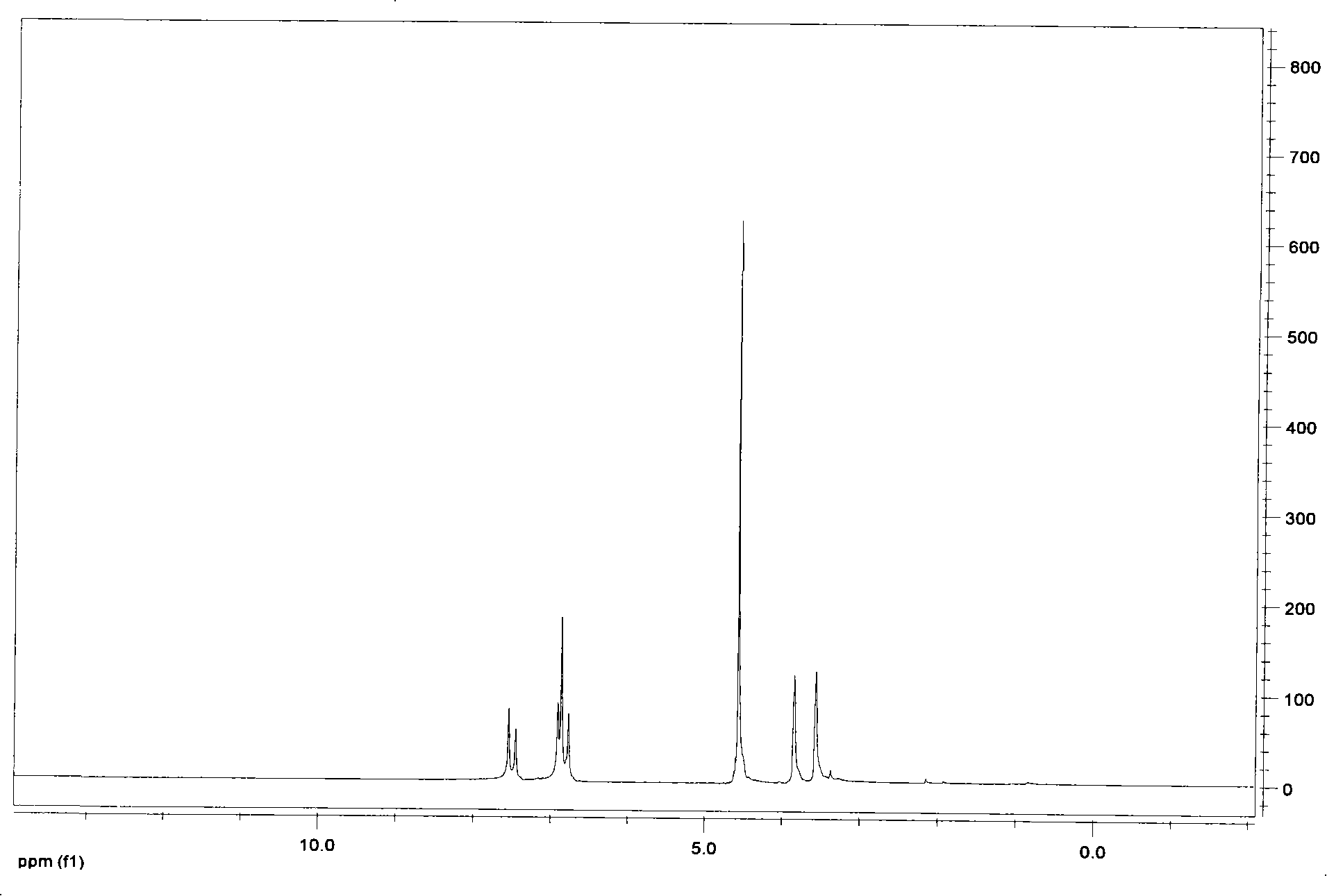
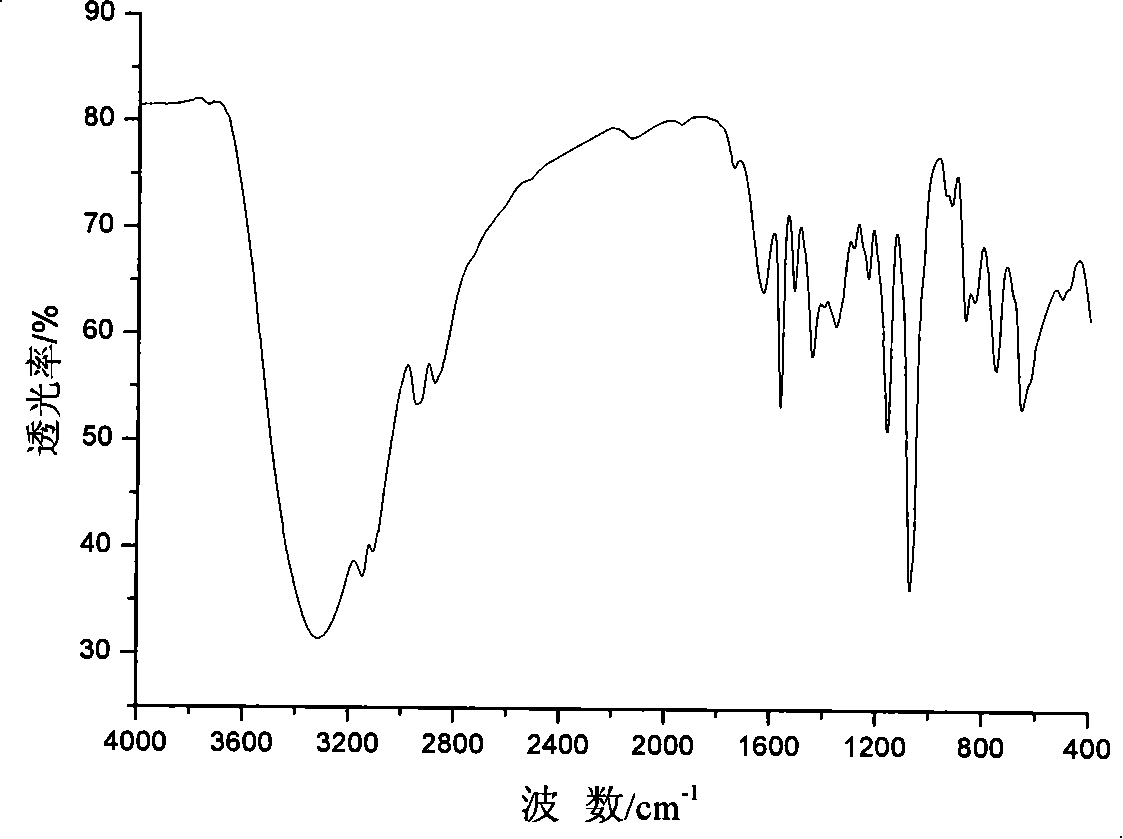
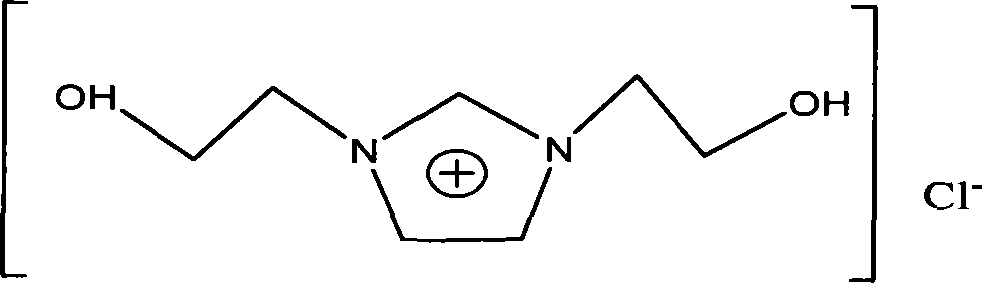
[0036] 1,3-bis(2-hydroxyethyl)imidazolium chloride ionic liquid——[hhim] + Cl - The synthesis: in equipped with magnetic stirring, reflux condenser, N 2 Add 100ml of absolute ethanol and 4.7g of metallic sodium to the 250ml three-necked flask of the catheter, and put the three-necked flask into an ice-water bath at -1 to 2°C to react for about 7 hours. After the reaction is complete, add 0.2mol of Imidazole, then slowly add 0.42mol of 2-chloroethanol (re-distilled under reduced pressure) with a dropping funnel, stir for 7h, take out the three-necked flask from the ice-water bath, react at 20°C for 40h, and filter to obtain Light yellow liquid, ethanol and unreacted 2-chloroethanol were evaporated under reduced pressure, washed several times with ethyl acetate, and the obtained light yellow sticky substance was obtained as a crude product for use.
[0037] 1,3-bis(2-hydroxyethyl)imidazolium chloride ionic liquid——[hhim] + Cl - Purification: take 2g[hhIm] + Cl - Dissolve in...
Embodiment 2
[0039] 1,3-bis(2-hydroxyethyl)imidazolium chloride ionic liquid——[hhim] + Cl - The synthesis: in equipped with magnetic stirring, reflux condenser, N 2 Add 100ml of absolute ethanol and 4.7g of sodium metal to the 250ml three-necked flask of the catheter, put the three-necked flask in an ice-water bath at -1 to 2°C, and react for about 8 hours. After the reaction is complete, add 0.2mol 0.41mol of 2-chloroethanol (re-distilled under reduced pressure when used) was slowly added dropwise with a dropping funnel, reacted for 7h, took out the three-necked flask from the ice-water bath, reacted at 30°C for 48h, and filtered to obtain The light yellow liquid was distilled off under reduced pressure to remove the methanol solvent and unreacted 2-chloroethanol, and washed several times with ethyl acetate to obtain a light yellow viscous product as a crude product for use.
[0040] 1,3-bis(2-hydroxyethyl)imidazolium chloride ionic liquid——[hhim] + Cl- Purification: take 2g[hhIm] + C...
Embodiment 3
[0042] 1,3-bis(2-hydroxyethyl)imidazolium chloride ionic liquid——[hhim] + Cl - The synthesis: in equipped with magnetic stirring, reflux condenser, N 2 Add 100ml of absolute ethanol and 4.7g of metallic sodium to the 250ml three-necked flask of the catheter, put the three-necked flask into an ice-water bath at -1 to 2°C, and react for about 7.5 hours. After the reaction is complete, add 0.2mol 0.42mol of 2-chloroethanol (re-distilled under reduced pressure when used) was slowly added dropwise with a dropping funnel, refluxed for 7h, and the three-necked flask was taken out from the ice-water bath, reacted at 50°C for 48h, and filtered to obtain The light yellow liquid was evaporated under reduced pressure to remove the methanol solvent and unreacted 2-chloroethanol, washed several times with ethyl acetate to obtain a light yellow sticky substance, and the crude product was obtained for use.
[0043] 1,3-bis(2-hydroxyethyl)imidazolium chloride ionic liquid——[hhim] + Cl - Pu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com