Use of medicament of geldanamycin derivative for preparing medicament for curing chemotherapy induction alopecia
A technology of geldanamycin and methoxygeldanamycin, which is applied in the field of geldanamycin derivatives, can solve problems such as unisolated geldanamycin-resistant strains, and achieve reliable and safe sex, reduce psychological stress, and improve the quality of life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
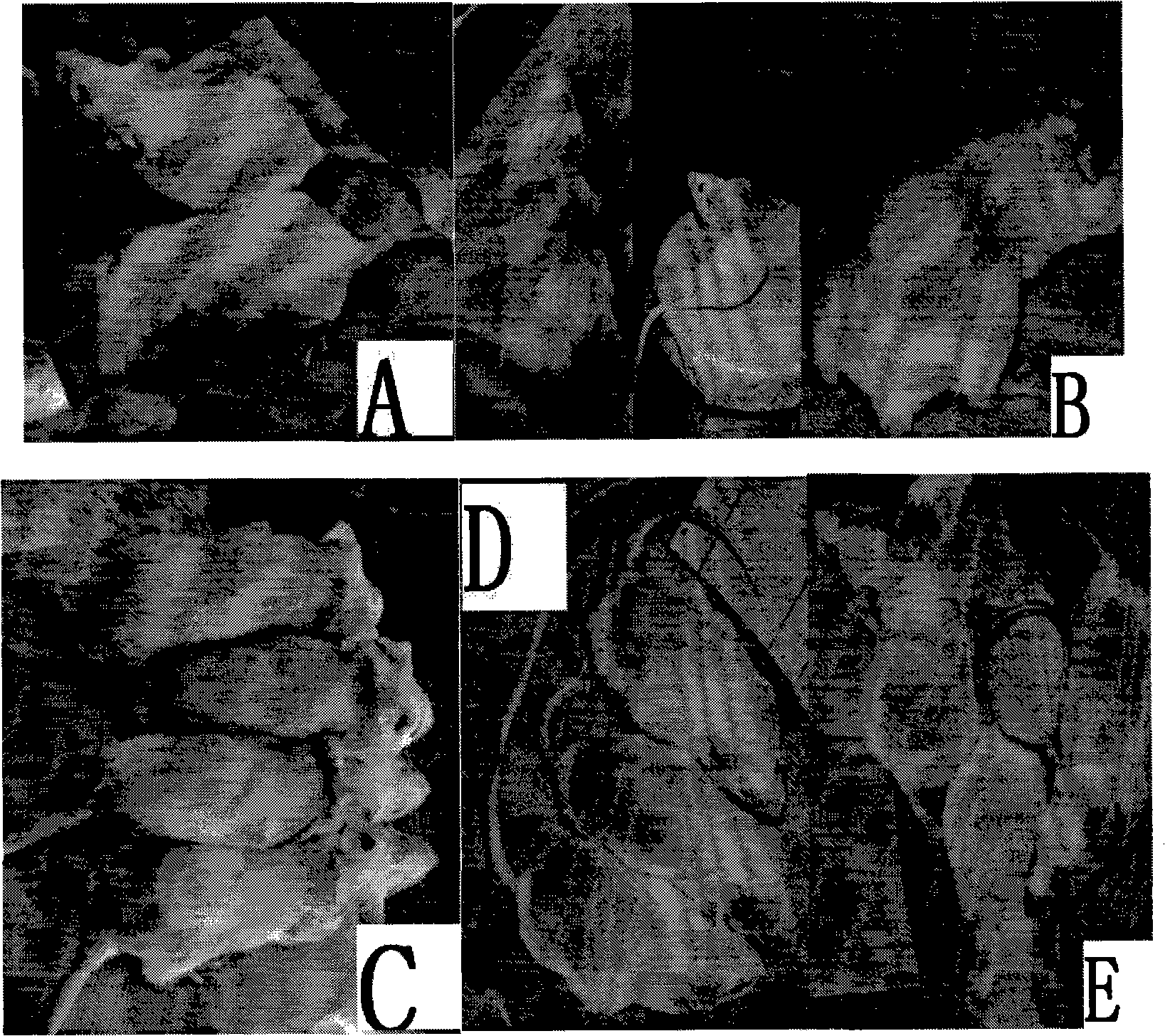
[0030] Embodiment: animal model and treatment of alopecia after chemotherapy
[0031] 1. Experimental materials
[0032] The experimental animals were SPF grade Km mice aged 4-6 weeks, provided by the Experimental Animal Center of Sun Yat-sen University, and raised at room temperature.
[0033] Cyclophosphamide and paclitaxel for injection were purchased from Sun Yat-sen University Cancer Hospital, and other commercially available products can also be used;
[0034] Therapeutic drug: 17-propenylamino-17-desmethoxygeldanamycin (17-AAG), purchased from SIGMA Company.
[0035] Others: physiological saline; chloral hydrate; water bath and other heating equipment.
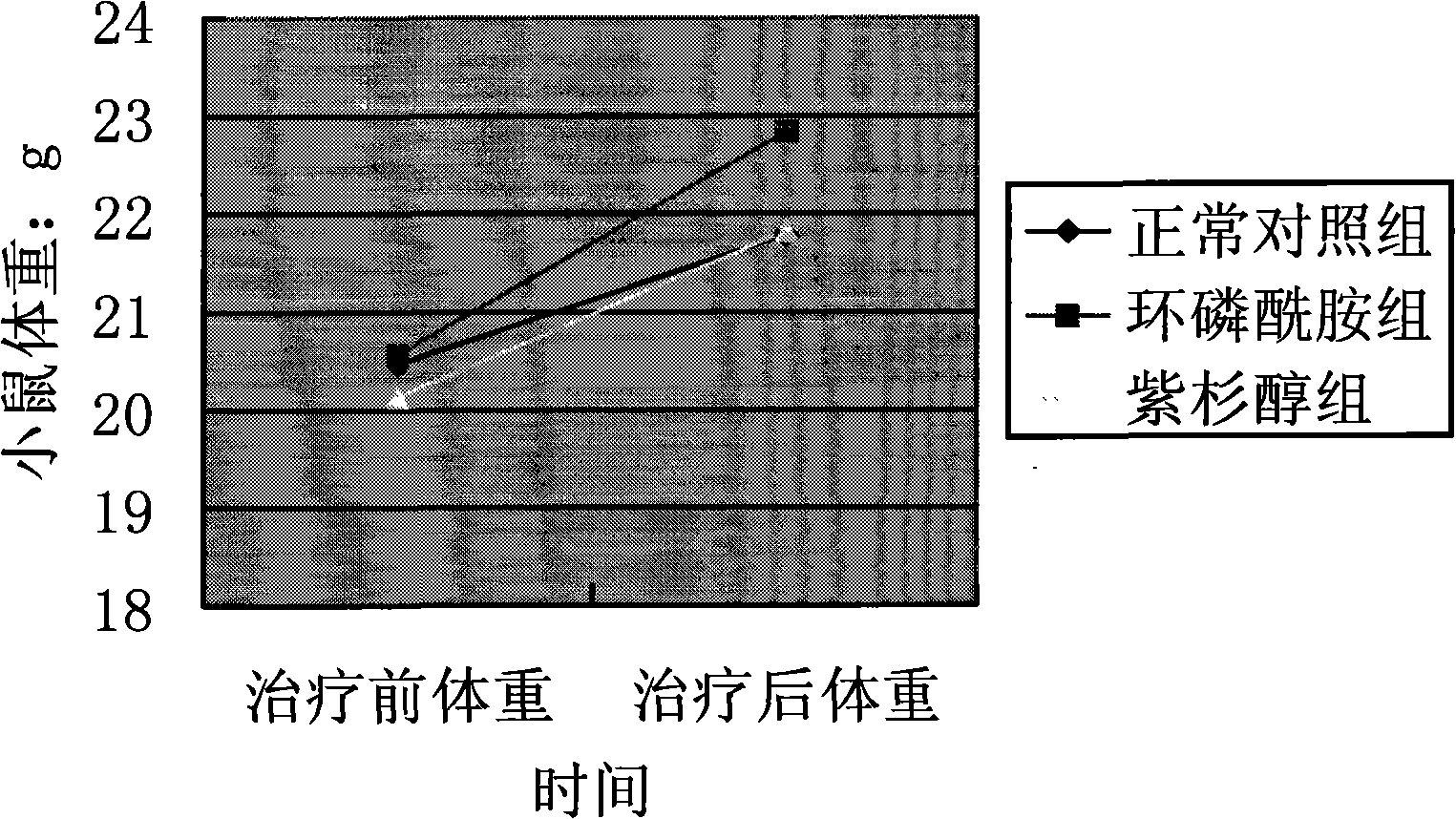
[0036] 2. Experimental methods and results
[0037] The experimental mice were randomly divided into five groups: control group, cyclophosphamide chemotherapy experimental group (no drug treatment), cyclophosphamide chemotherapy + drug treatment group, paclitaxel chemotherapy group (no drug treatment), paclitaxel che...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com