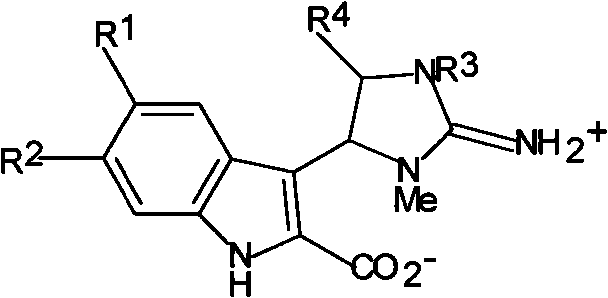
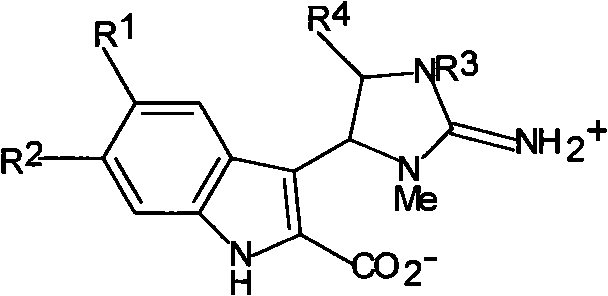
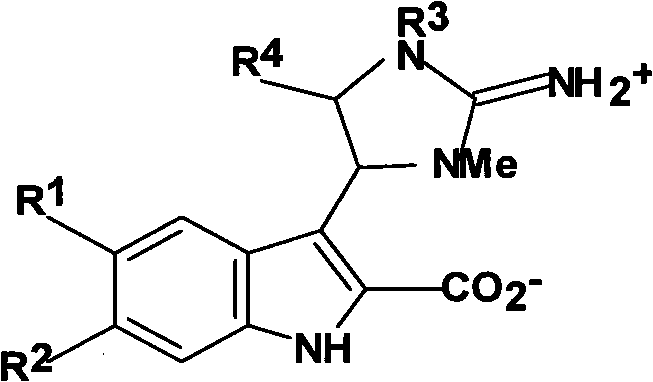
Antineoplastic activity marine indole alkaloids substances, and preparation method and application thereof
A technology for indole alkaloids and anti-tumor activity, applied in the field of medicine, can solve the problem that the activity data of human lung cancer cell A-549 is not specific, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Trachycladindole A(1)
[0035] Sponge Trachycladus sp.1.0kg, soaked in 4.0kg ethanol for 96 hours, repeated extraction twice, filtered, combined extracts, concentrated under reduced pressure to obtain extract (1.8g). Suspend the entire extract in water, add petroleum ether to degrease twice, add n-butanol (1:1, V / V) to the aqueous phase for extraction, evaporate the n-butanol extract to dryness under reduced pressure to obtain 300 mg of extract, and pass through Sephadex LH 20 Column chromatography separated to obtain five components A (60 mg), B (150 mg), C (50 mg), D (30 mg), and E (10 mg).
[0036] Among them, component A is separated and purified by RP-3 semi-preparative reversed-phase high-performance liquid chromatography, detected by a diode array ultraviolet detector, and gradient eluted with methanol-water (10-100% methanol) to obtain Trachycladindole A (that is, the above compound 1 ), light yellow powder (8mg), the structure was identified by high-resolution...
Embodiment 2
[0039] Trachycladindole B(2)
[0040] Component B in Example 1 was separated by RP-3 semi-preparative reversed-phase high performance liquid chromatography, and methanol-water (10-100% methanol) gradient elution was used to obtain components B-1, B-2, and B-3. Wherein, B-1 further uses RP-CN semi-preparative reversed-phase high performance liquid chromatography, diode array ultraviolet detector detection, acetonitrile-water solvent system separation to obtain Trachycladindole B (that is, the above compound 2), light yellow powder (4mg), The structure is identified by high-resolution mass spectrometry, nuclear magnetic resonance and other spectra, and its spectral data:
[0041] [α] D 20 +8.9(c 0.94, MeOH); UV(MeOH)λ max nm(logε)225(3.1), 296(2.6).HRESI(+)MS m / z 373.0268([M+Na] + , C 14 h 14 NaBrN 4 o 2 + requires 373.0271), 375.0262 (C 14 h 14 Na 81 BrN 4 o 2 + ). 1 Hnmr (CDCl 3 , 600MHz) δppm 2.75 (3H, s), 3.10 (3H, s), 3.70, 4.06 (each 1H, dd, J = 8.0, 8.0Hz...
Embodiment 3
[0043] Trachycladindole C(3)
[0044] Component B-2 obtained in Example 2 was further separated by RP-CN semi-preparative reversed-phase high performance liquid chromatography and acetonitrile-water solvent system to obtain Trachycladindole C (i.e. the above compound 3), light yellow powder (7mg), The structure is identified by high-resolution mass spectrometry, nuclear magnetic resonance and other spectra, and its spectral data:
[0045] [α] D 20 +2.9(c 1.31, MeOH); UV(MeOH)λ max nm(log)225(3.24), 245(3.04), 313(3.12), 320(3.10).HRESI(+)MS m / z 353.0253([M+H] + , C 13 h 14 BrN 4 o 3 + requires 357.0244), 355.0230 (C 13 h 14 81 BrN 4 o 3 + ). 1 Hnmr (CDCl 3 , 600MHz) δppm 2.70 (3H, s), 3.68, 4.04 (each 1H, dd, J = 8.0, 8.0Hz), 6.36 (1H, brs), 7.00 (1H, s), 7.55 (1H, brs). 13 Cnmr (CDCl 3 , 150MHz) δppm 29.7(q), 48.3(t), 58.9(d), 99.2(d), 106.0(s), 113.0(s), 121.2(s), 124.2(d), 135.4(s), 137.6 (s), 152.3(s), 160.3(s), 169.2(s).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com