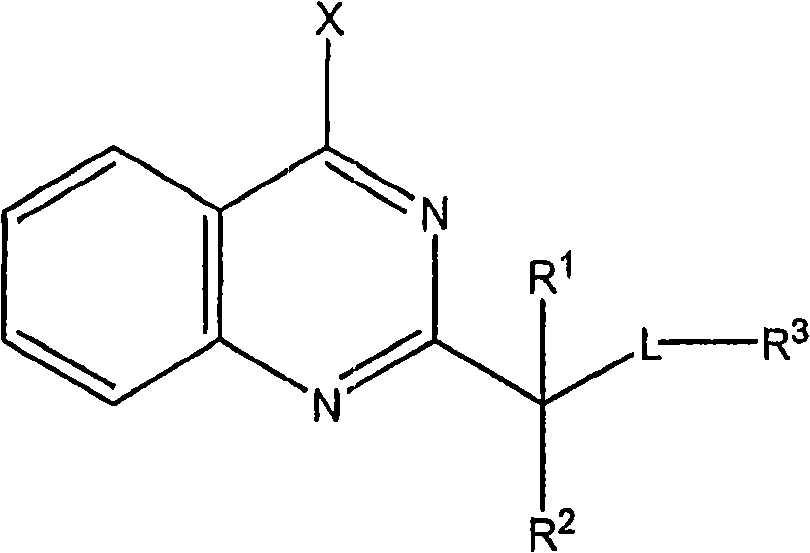
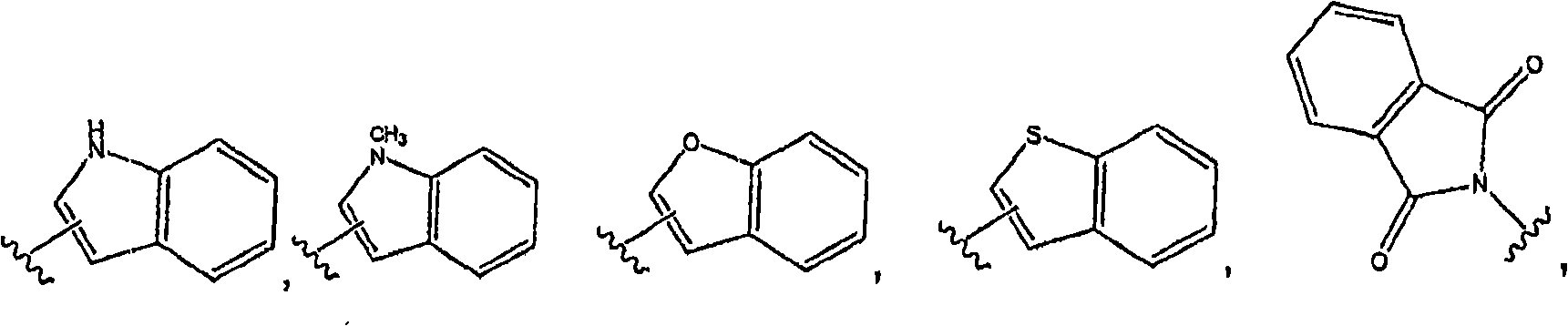
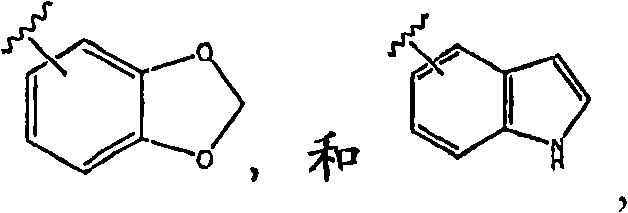
Quinazoline derivatives useful in cancer treatment
A compound and solvate technology, which is applied in the field of quinazoline derivatives for the treatment of cancer, can solve the problems of loss of DNA binding and sequence-specific transcription regulation functions, conformational changes in domains, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 2
[0346] 5-bromo-2-[chloro-(2,4-dichlorophenyl)methyl]pyridine
[0347]
[0348] A. 2,4-dichloro-N-methoxy-N-methylbenzamide
[0349]
[0350] 2,4-Dichlorobenzoyl chloride (11.59 g, 7.76 mL, 55.3 mmoles) and N,O-dimethylhydroxylamine hydrochloride (4.91 g, 50.3 mmoles) were dissolved in dry dichloromethane (550 mL) , and the mixture was cooled to 0 °C under argon. Anhydrous pyridine (8.76 g, 8.96 mL, 110.6 mmoles) was added dropwise to the stirred solution at 0°C, and the mixture was stirred at 0°C for 6 hours. The mixture was evaporated to dryness and the residue was partitioned between ether-dichloromethane (1:1) and brine. The organic layer was dried (MgSO 4 ), filtered and evaporated to dryness. The residue was chromatographed on a silica gel column (60×5 cm) with 1% (10% concentrated NH 4 OH / methanol)-dichloromethane as eluent afforded the title compound (11.78 g, 100%) as a colorless oil: ESMS: m / z 234.0 (MH + ); measured value:
[0351] C, 46.16; H, 3.55; Cl,...
preparation Embodiment 3
[0367] A. (6-bromopyridin-3-yl)-(2,4-dichlorophenyl)methanone
[0368]
[0369] 2,5-Dibromopyridine (10.8 g, 45.6 mmoles) was dissolved in anhydrous ether (541 mL), and the mixture was stirred at -78°C under argon. 2.5M n-BuLi / hexane (21.5 mL, 54.7 mmoles) was added dropwise at -78°C over 10 minutes, and the mixture was stirred at -78°C for 40 minutes. 2,4-Dichloro-N-methoxy-N-methylbenzamide (10.64 g, 45.61 mmoles) (prepared as described above in Preparative Example 2, Step A) was dissolved in anhydrous diethyl ether over 10 minutes. (8 ml) was added dropwise to the stirred solution, and the mixture was stirred at -78°C for 1 hour. The mixture was allowed to warm to -10°C. Add saturated NH 4 Cl aqueous solution (108 mL), the mixture was stirred and allowed to warm to 25 °C. The ether layer was separated and dried (MgSO 4 ), filtered and evaporated to dryness. The residue was chromatographed on a silica gel column (30 x 5 cm) with 2% ethyl acetate / hexane as eluent to ...
preparation Embodiment 4
[0380] 5-bromo-2-[chloro-(3,5-dichlorophenyl)methyl]pyridine
[0381]
[0382] A. 3,5-dichloro-N-methoxy-N-methylbenzamide
[0383]
[0384] Dissolve 3,5-dichlorobenzoyl chloride (10.0 g, 47.7 mmoles) and N, O-dimethylhydroxylamine hydrochloride (4.23 g, 43.4 mmoles) in anhydrous dichloromethane (475 mL) under argon The mixture was cooled to 0°C under air. Anhydrous pyridine (7.55 g, 7.79 mL, 95.5 mmoles) was added dropwise to the stirred solution at 0°C, and the mixture was stirred at 0°C for 5 hours. The mixture was evaporated to dryness and the residue was partitioned between ether-dichloromethane (1:1) and brine. The organic layer was dried (MgSO 4 ), filtered and evaporated to dryness. The residue was chromatographed on a silica gel column (60×5 cm) with 0.75% (10% concentrated NH 4 OH / methanol)-dichloromethane as eluent afforded 3,5-dichloro-N-methoxy-N-methylbenzamide (9.66 g, 95%) as a colorless oil: FABMS: m / z 234.2 (MH + ); HRFABMS: m / z 234.0090 (MH + )...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com