Antibodies for the treatment of cancers
An antibody and monoclonal antibody technology, applied in the field of anti-EGFR immunoconjugates, can solve problems such as cancer chemotherapy obstacles, malignant cell destruction, and inability to recognize tumor cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
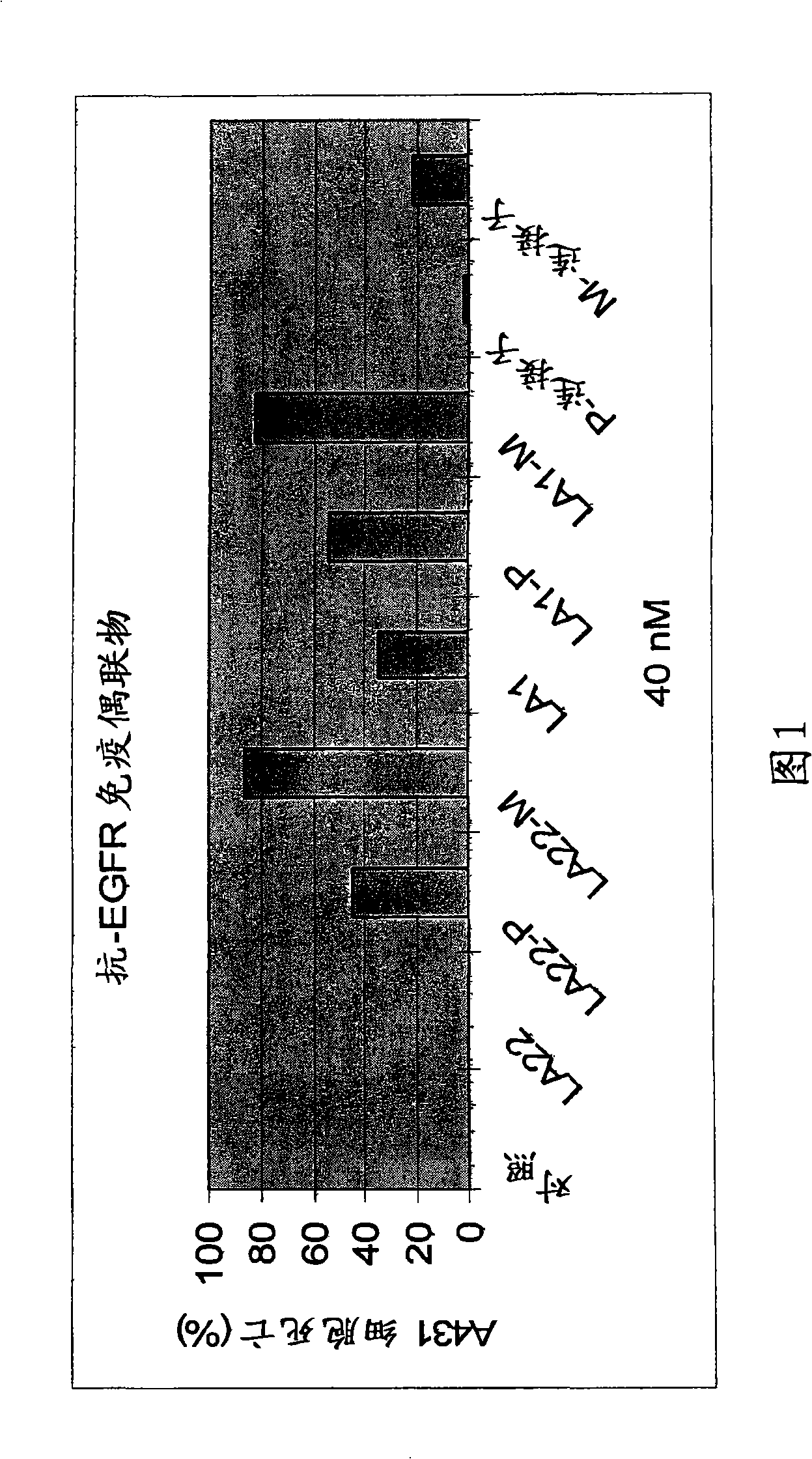
[0057] A431 cells (human epidermoid carcinoma cell line (ATCC No. CRL-1551)) were seeded in a 96-well plate containing DMEM-10% FBS at an amount of 2000 cells / well. After 4 hours, the control medium, mAb LA22 (40nM), LA22-PYM immunoconjugate (LA22-P, 40nM), LA22-MMC immunoconjugate (LA22-M, 40nM), mAb LA1 (40nM) , LA1-PYM immunoconjugate (LA1-P, 40nM), LA1-MMC immunoconjugate (LA1-M, 40nM), PYM-SPDP (P-linker, 40nM), or MMC-SPDP (M- Linker, 40 nM) was added to the cell culture. After 4 days, the number of cells in each well was determined by MTT assay (Mosmann, J. Immunol. Methods, 65:55-63 (1983)). The numbers are expressed as percent cell death (Figure 1).
[0058] As shown in Figure 1, no significant cell death was observed with 40 nM anti-EGFR mAb LA22 applied alone, while LA22-PYM and LA22-MMC caused 42% and 84% cell death, respectively. At the same dose (4OnM), anti-EGFR mAb LA1 alone caused 35% cell death. Increased cell killing activity was observed for LA1-PYM and...
Embodiment 2
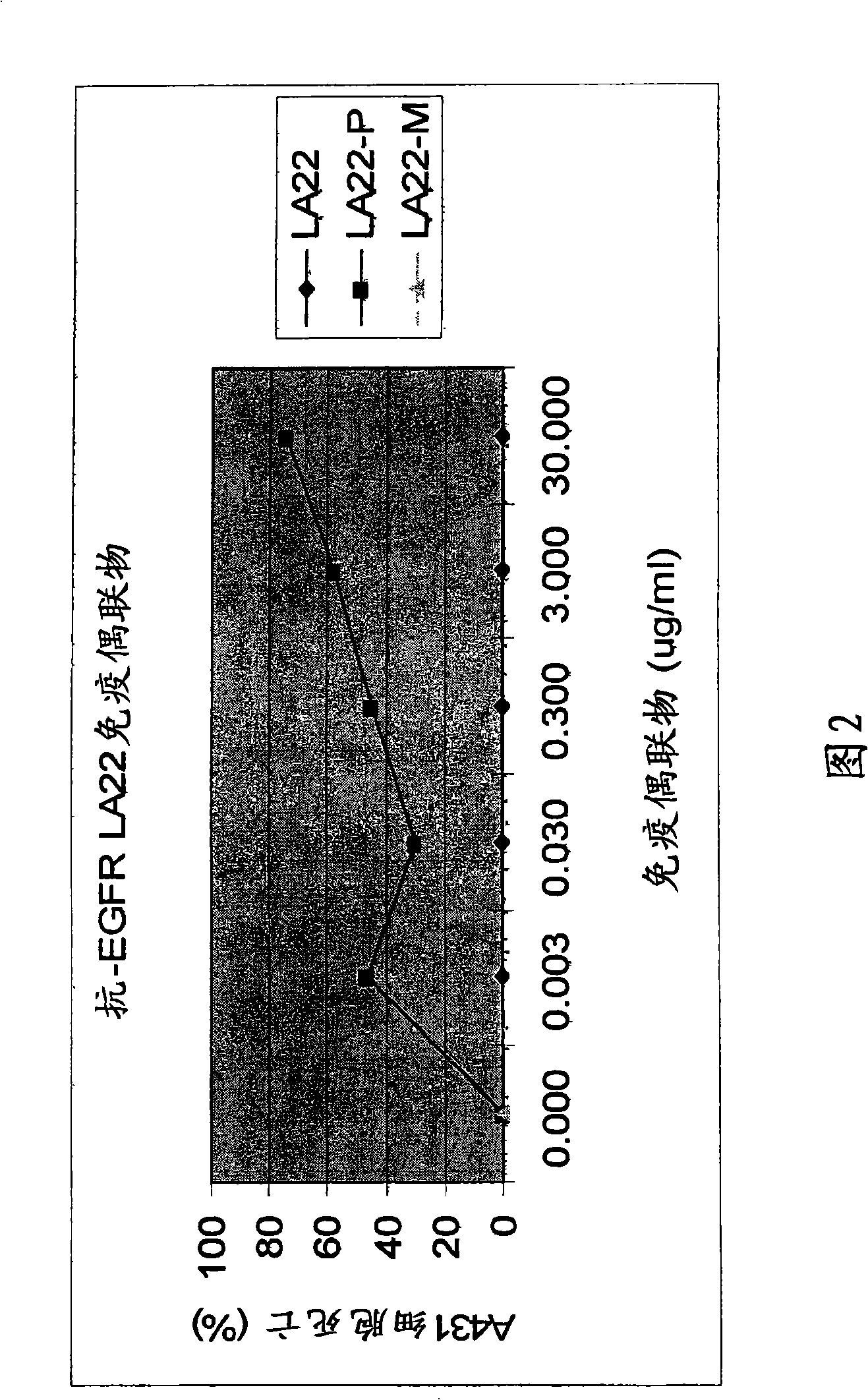
[0060] In order to evaluate the concentration dependence of the above-mentioned in vitro cytotoxicity of LA22 antibiotic immunoconjugates, 4 hours after the cells were inoculated in a 96-well plate of DMEM-10% FBS at an amount of 2000 cells / well, different amounts of LA22, LA22-PYM and LA22-MMC were added to A431 cell cultures. After 4 days, the number of cells per well was determined by MTT assay. The numbers are expressed as percent cell death (Figure 2). Figure 2 illustrates that anti-EGFR mAb LA22-PYM or LA22-MMC caused A431 human cancer cell death in a dose-dependent manner.
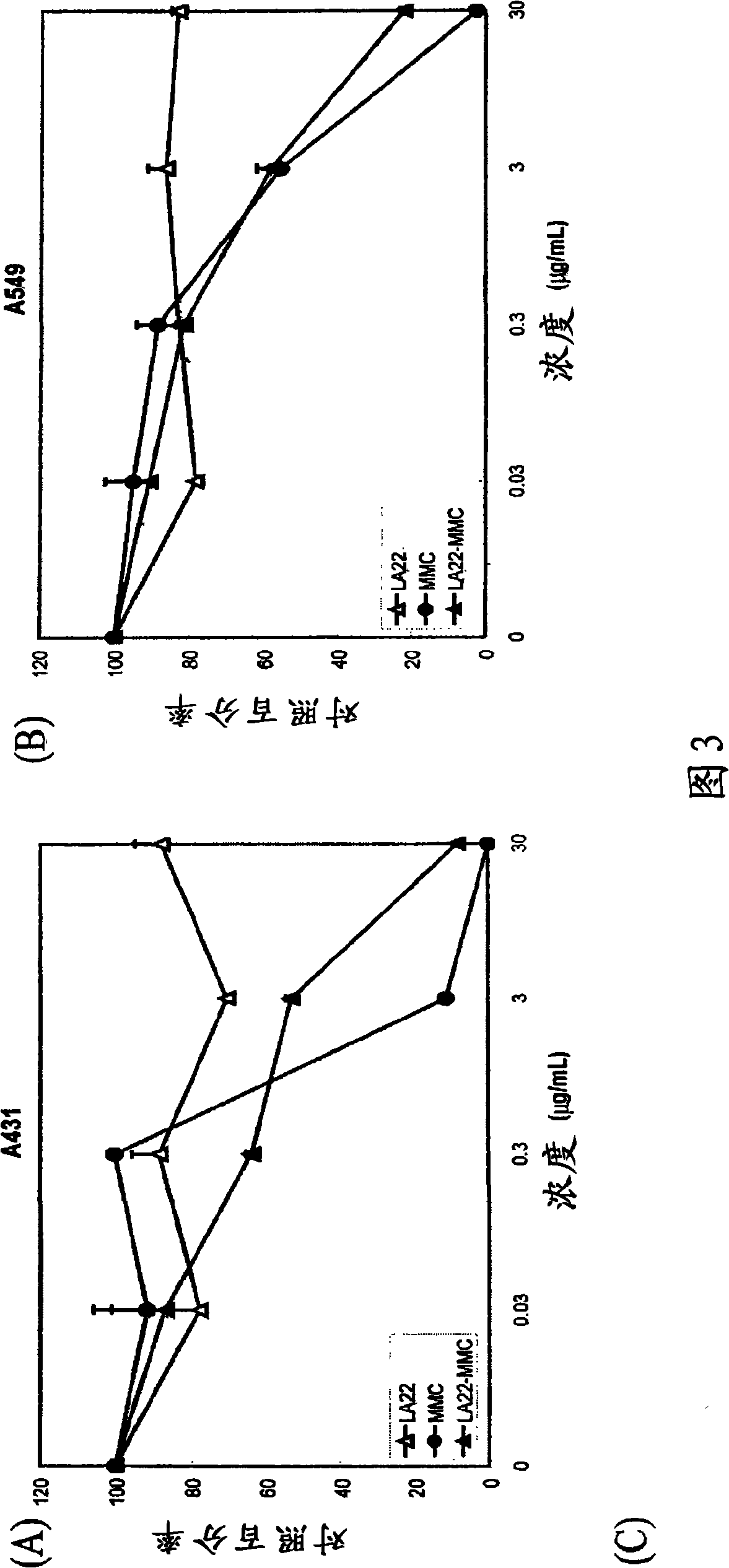
[0061] Another LA22-MMC cytotoxicity assay was performed along with additional controls. A431 cells and human lung adenocarcinoma A549 cells (ATCC No.CCL-185) were inoculated (2×10 3 cells / well, 100 μL / well) in a 96-well cell culture plate (Corning, Corning, NY) in the presence of DMEM medium and 10% FBS, at 37°C and 5% CO 2 conditions for 4 hours. Add MMC, naked mAb LA22, or LA22-MMC immunocon...
Embodiment 3
[0064] Likewise, in vitro cytotoxicity assays using LA1 antibiotic immunoconjugates were evaluated in a similar manner. A431 cells were seeded in a 96-well plate in DMEM-10% FBS at an amount of 2000 cells / well. After 4 hours, different amounts of LA1, LA1-PYM or LA1-MMC were added to the cell culture. After 4 days, the number of cells per well was determined by MTT assay. The numbers are expressed as percent cell death (Figure 4). As shown in Figure 3, mAb LA1 and its immunoconjugates induced A431 human cancer cell death in a dose-dependent manner. Conjugation with pingyangmycin or mitomycin C significantly increased the cell killing potency of mAb LA1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com