Preparation of potassium platinochloride
A technology of potassium chloroplatinite and potassium chloroplatinate, applied in chemical instruments and methods, sodium/potassium compounds, ruthenium/rhodium/palladium/osmium/iridium/platinum compounds, etc., which can solve the lengthy preparation process and concentration limitations , high production cost and other issues, to achieve the effect of good operating environment, low production cost and short preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
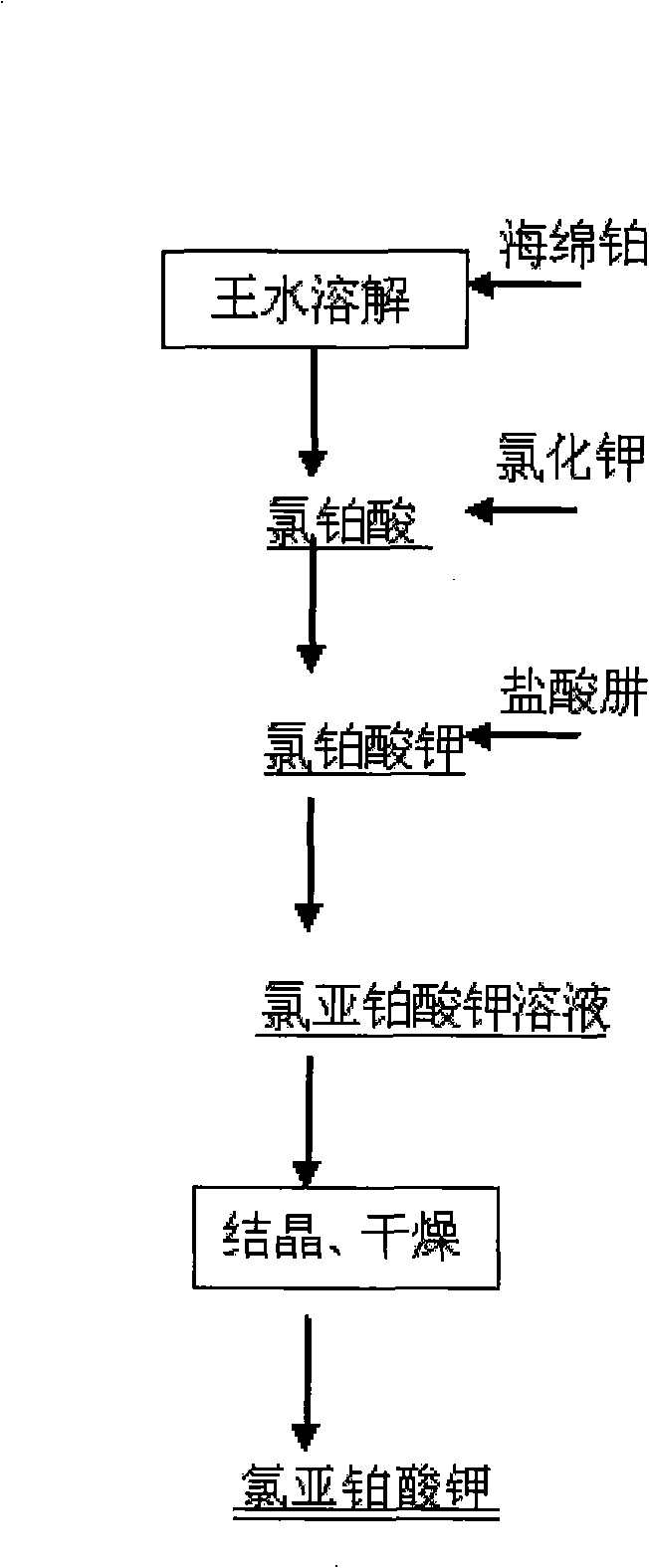
[0012] A preparation method of potassium chloroplatinite, the preparation process is as follows: using sponge platinum as raw material to prepare chloroplatinic acid solution and potassium chloride solution to prepare potassium chloroplatinate, and then using hydrazine hydrochloride as reducing agent to reduce chloroplatinic acid Potassium for the preparation of pharmaceutical intermediates potassium chloroplatinite. Include the following steps:
[0013] a) Preparation of potassium chloroplatinate: Weigh a certain amount of sponge platinum, wash it, put it into a beaker, add an appropriate amount of aqua regia in batches, heat properly, and the reaction process is based on the principle of uniform bubbling. After the reaction was stopped, concentrated hydrochloric acid was added dropwise under heating and boiling to catch the nitrate until the system no longer emitted brown nitrogen oxides, and then an appropriate amount of water was added to catch the acid. After filtering, ...
Embodiment 1
[0020] Weigh 100g of platinum sponge, wash it, put it into a beaker, add an appropriate amount of aqua regia, and heat it appropriately. The reaction process is based on the principle of uniform bubbling. After the reaction was stopped, the obtained solution was concentrated until crystals were precipitated, concentrated hydrochloric acid was added dropwise to drive the nitrate until the system no longer emitted brown nitrogen oxides, then concentrated until crystals were precipitated, and an appropriate amount of deionized water was added to drive the acid. Filtration, and the filtrate was prepared into a 50g / L solution for later use.
[0021] 80.0 g of potassium chloride was weighed, made into a 20% solution, filtered, and the filtrate was used for later use. The above chloroplatinic acid solution was taken and added dropwise to the potassium chloride solution under stirring, and the solution appeared yellow precipitation. After the dropwise addition, continued stirring for ...
Embodiment 2
[0025] The specific implementation rules are the same as Example 1, the difference is that the input amount of sponge platinum is 500g, the chloroplatinic acid is prepared into a solution of 100g / L, the potassium chloride consumption is 401g, and the solution is made into 20%, and the potassium chloroplatinate is 1200g, 8000ml of distilled water was added, and the amount of reducing agent hydrazine hydrochloride was 136g. The obtained product was sampled for full elemental analysis and XRD detection, platinum content: 46.2%, potassium content: 17.6%, and no compounds other than potassium chloroplatinite were detected. The product yield was 95.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com