Dihydrogen phosphate salt of a prostaglandin d2 receptor antagonist
A compound and drug technology, applied in the field of dihydrogen phosphate, can solve problems that are not specifically disclosed
Inactive Publication Date: 2008-10-15
AVENTIS PHARMA INC
View PDF4 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
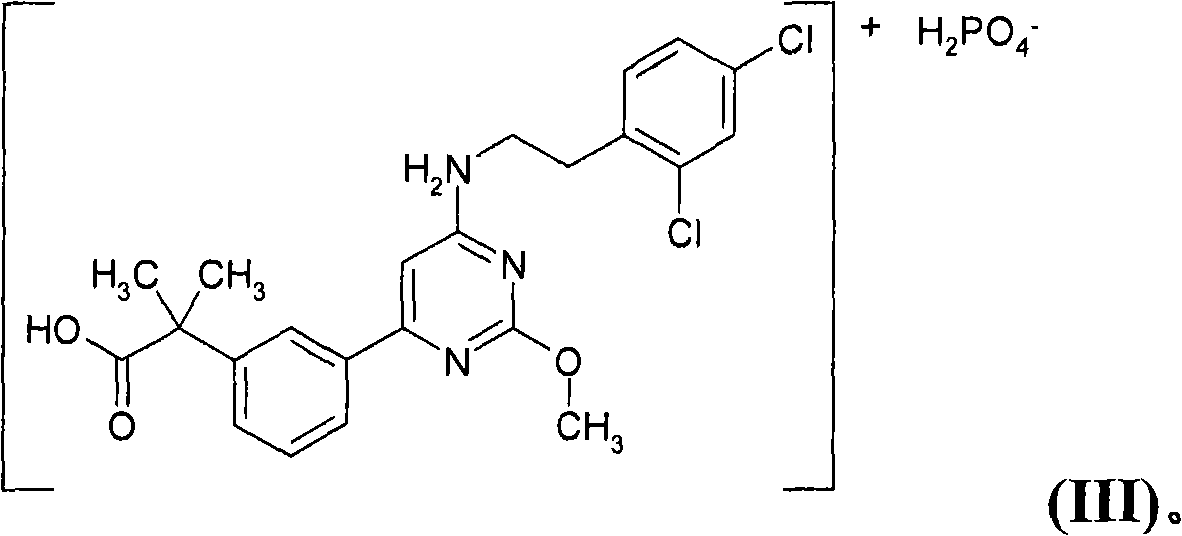
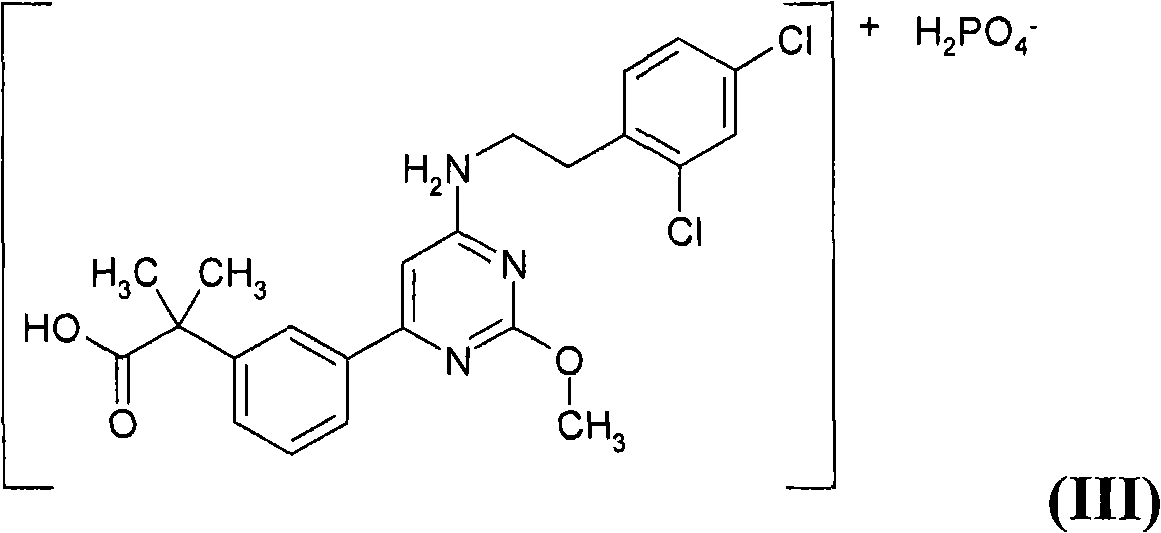
However, the '732 publication does not specifically disclose that 2-(3-{6-[2-(2,4-dichlorophenyl)ethylamino]-2-methoxypyrimidin-4-yl}phenyl)- Dihydrogen Phosphate of 2-Methylpropionic Acid
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment approach
Embodiment
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Login to View More
Abstract
The present invention is directed to the dihydrogen phosphate salt of 2-(3-{6-[2-(2,4-dichloro-phenyl)-ethylamino]-2-methoxy-pyrimidin-4-yl}-phenyl)-2-methyl-propionic acid of Formula (III), a pharmaceutical composition comprising a pharmaceutically effective amount of the compound of Formula (III), and a pharmaceutically acceptable carrier; and a method of treating a patient suffering from a PGD2-mediated disorder including, but not limited to, allergic disease (such as allergic rhinitis, allergic conjunctivitis, atopic dermatitis, bronchial asthma and food allergy), systemic mastocytosis, disorders accompanied by systemic mast cell activation, anaphylaxis shock, bronchoconstriction, bronchitis, urticaria, eczema, diseases accompanied by itch (such as atopic dermatitis and urticaria), diseases (such as cataract, retinal detachment, inflammation, infection and sleeping disorders) which is generated secondarily as a result of behavior accompanied by itch (such as scratching and beating), inflammation, chronic obstructive pulmonary diseases, ischemic reperfusion injury, cerebrovascular accident, chronic rheumatoid arthritis, pleurisy, ulcerative colitis and the like.
Description
Dihydrogen Phosphate Salt of Prostaglandin D2 Receptor Antagonist field of invention Large-scale production of pharmaceutical compositions can pose many challenges to chemists and chemical engineers. While many of these challenges involve the handling of large quantities of reagents and the control of large-scale reactions, handling of the final product presents special challenges related to the nature of the final active product itself. Such a product must not only be high yielding, stable and easy to isolate, but it must have properties suitable for the type of pharmaceutical formulation that may ultimately be used. The stability of active ingredients of pharmaceutical formulations must be considered at every step of the production process, including synthesis, isolation, bulk storage, drug formulation and long-term formulation. Each of these steps can be affected by various environmental conditions such as temperature and humidity. The pharmaceutically active substance ...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): C07D239/46C07D239/47A61K31/505
CPCC07D239/47A61P1/00A61P11/00A61P11/06A61P11/08A61P17/00A61P17/02A61P17/04A61P19/02A61P25/20A61P27/00A61P27/12A61P27/14A61P29/00A61P31/00A61P35/00A61P37/00A61P37/08A61P9/10C07D239/46A61K31/505
Inventor B·朗之万E·奥顿D·谢勒
Owner AVENTIS PHARMA INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com