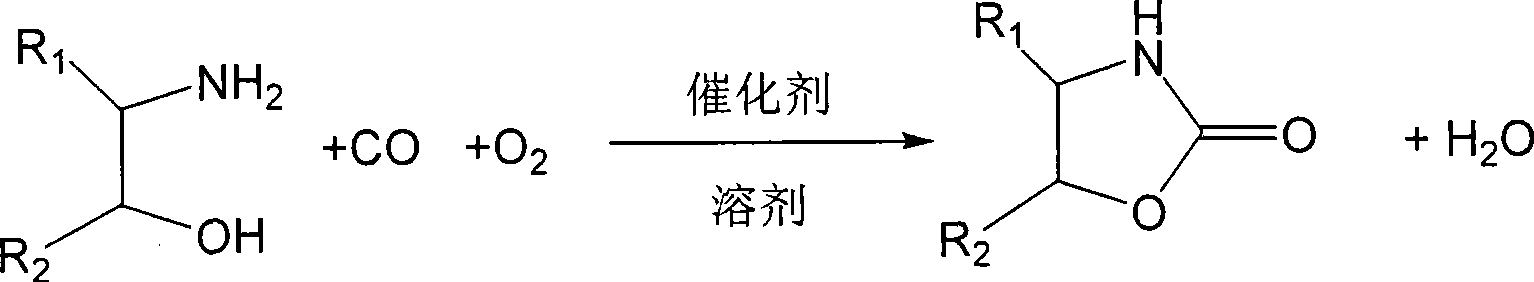
Method for synthesizing oxazoline-2-ketone
A technology for synthesizing oxazoline and oxazoline, which is applied in organic chemistry and other fields, can solve the problems of difficult separation of catalysts and products, and achieve the effects of large-scale industrial production, low corrosion, and easy subsequent separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Add 10ml of acetonitrile, 0.61g (10mmol) of ethanolamine, 0.0395g (0.5mmol) of Se into a three-necked flask, put it in an oil bath that has been heated to 30°C, stir and react with carbon monoxide for about 15 minutes to approximately equal the volume of CO 10 Oxygen was introduced at a rate of %, and the reaction was stirred for 8h. Stop CO, continue to oxidize with oxygen for 2 hours, filter and recover the catalyst, and evaporate the solvent in vacuum to obtain the crude product. After purification by recrystallization, the product is weighed to obtain 0.84 g of oxazoline-2-one. The first single pass yield is 93. % (Based on ethanolamine).
Embodiment 2
[0028] The organic solvent is dimethylformamide, and the experimental method and steps are the same as those in Example 1. The first single pass yield is 85% (calculated as ethanolamine).
Embodiment 3
[0030] The organic solvent is pyridine, and the experimental method and steps are the same as in Example 1. The first single-pass yield is 93% (calculated as ethanolamine).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com