A method for reclaiming piperazine after the piperazine reduction reaction in the preparation of quinolone medicines
A quinolone and post-reaction technology, applied in the field of chemical pharmaceuticals, can solve problems such as unsuitable for industrial production and use, achieve great application value, reduce waste gas, and improve the effect of operating environment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
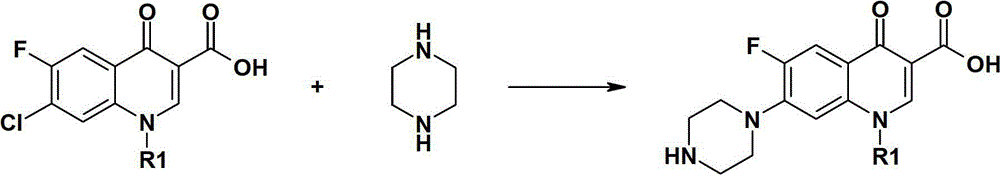
[0020] Take 30 grams (0.11 moles) of cyclopropanecarboxylic acid (i.e. 1-cyclopropyl-6-fluoro-7-chloro-4-oxo-1,4-dihydro-3-quinoline carboxylic acid), according to the literature Method (Tetra. Lett. 1996, 37, 6785, etc.) and 60 grams of anhydrous piperazine (0.70 moles) were reacted with piperazine. During the reaction, 32 grams of purified water and 3 grams of aluminum acetate were added, and reflux at 110 ° C for 8 hours to prepare Obtained piperazine reaction solution, after the reaction, add 35 grams of 30% sodium hydroxide aqueous solution, stir and react for 0.5 hour, then reduce the pressure to -0.01Mpa, heat up to 115-120°C to recover piperazine, and after recovery for 2 hours, basically No material was evaporated, and the vacuum was increased to -0.09MPa, and recovered for 15 minutes. The recovered material system became powdery, and 115.4 grams of recovered material were obtained, which contained 48.69 grams (0.57 moles) of piperazine, and the recovery rate was 96.6%...
Embodiment 2
[0024] Take 30 grams (0.11 moles) of cyclopropanecarboxylic acid (i.e. 1-cyclopropyl-6-fluoro-7-chloro-4-oxo-1,4-dihydro-3-quinoline carboxylic acid), according to the literature Methods (Tetra.Lett.1996, 37, 6785, etc.) react with anhydrous piperazine 60 grams (0.70 moles) to prepare the piperidine reaction solution, add 30% potassium hydroxide aqueous solution 32 grams, stir for 0.5 hours, and then Reduce the pressure to -0.02Mpa, raise the temperature to recover piperazine, after recovering for 1.5 hours, basically no material is evaporated, increase the vacuum to -0.09MPa, recover for 30 minutes, the system becomes powdery, and obtain 112.7 grams of recovered product, which contains 45.71 g of piperazine g (0.55 mol), the recovery rate was 90.1%.
[0025] MS(ESI):m / z=87[M+H + ] confirmed that the recovery was piperazine;
[0026] The recovered materials were salted and refined according to the literature method to obtain 31.04 g of ciprofloxacin hydrochloride finished pr...
Embodiment 3
[0028] Take 30 grams (0.11 moles) of cyclopropanecarboxylic acid (i.e. 1-cyclopropyl-6-fluoro-7-chloro-4-oxo-1,4-dihydro-3-quinoline carboxylic acid), according to the literature Method (Tetra.Lett.1996, 37, 6785, etc.) reacted with anhydrous piperazine 60 grams (0.70 moles) to prepare the piperidine reaction solution, added 48 grams of 50% sodium carbonate aqueous solution, stirred for 0.5 hours, and then reduced Press down to -0.03Mpa, raise the temperature to recover piperazine, after recovering for 1.5 hours, basically no material is evaporated, increase the vacuum to -0.09MPa, recover for 15 minutes, the system becomes powdery, and obtain 117.2 grams of recovered product, which contains 47.62 grams of piperazine (0.55 mol), the recovery rate was 93%.
[0029] MS(ESI):m / z=87[M+H + ] confirmed that the recovery was piperazine;
[0030] The recovered materials were salted and refined according to the literature method to obtain 31.04 g of ciprofloxacin hydrochloride finish...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com