A method for simultaneously preparing high-purity difluorophosphoric acid and high-purity lithium difluorophosphate
A technology of lithium difluorophosphate and difluorophosphoric acid, applied in chemical instruments and methods, phosphorus compounds, phosphorus halides/oxyhalides, etc., can solve the problems of difficult removal, many by-products, high energy consumption, etc., and avoid product hydrolysis , High conversion rate, energy saving effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
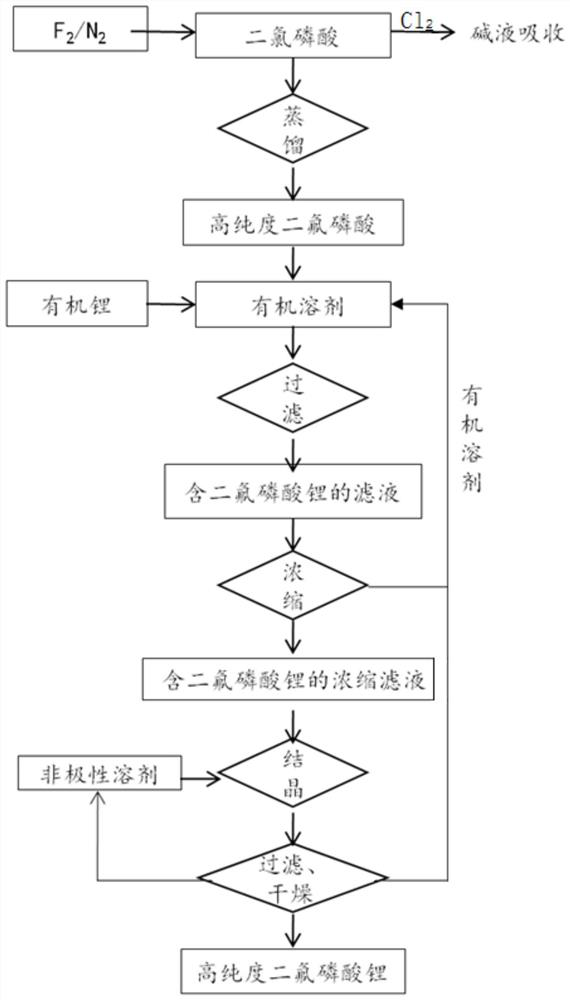
Image
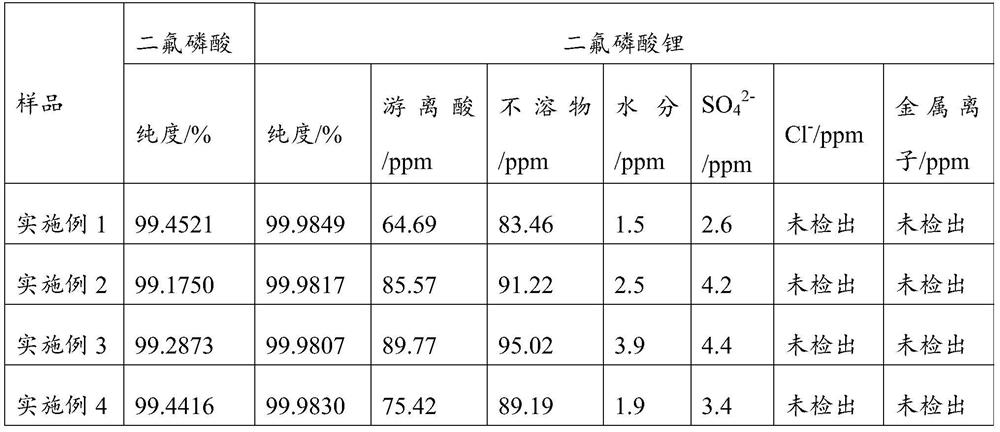
Examples
Embodiment 1
[0071] This example provides a method for simultaneously preparing high-purity difluorophosphoric acid and high-purity lithium difluorophosphate, which specifically includes the following steps:
[0072] (1) Nitrogen atmosphere, at 20°C, from the bottom at a rate of 450mL / min, into a 500mL three-necked flask filled with 135g of dichlorophosphoric acid, feed a 20% fluorine-nitrogen mixture with a mass fraction of fluorine gas, and after 322min, The system was purged with nitrogen; the temperature of the reaction system was raised to 110° C. for distillation, and 91.8 g of high-purity difluorophosphoric acid fractions were collected, with a yield of 90%.
[0073] (2) Under nitrogen atmosphere, drop 91.8g of high-purity difluorophosphoric acid into a solution prepared with 34.2g of lithium methoxide and 195g of methanol (water content ≤ 10ppm) at -5 to 15°C; heat the system to 25°C for 2 hours , and filtered to obtain the filtrate.
[0074] (3) Under the pressure of -0.1MPa, hea...
Embodiment 2
[0080] This example provides a method for simultaneously preparing high-purity difluorophosphoric acid and high-purity lithium difluorophosphate, which specifically includes the following steps:
[0081] (1) Nitrogen atmosphere, at 20°C, from the bottom at a rate of 500mL / min, into a 500mL three-necked flask filled with 150g of dichlorophosphoric acid, feed a 20% fluorine-nitrogen mixture with a mass fraction of fluorine gas, and after 331min, The system was purged with nitrogen; the temperature of the reaction system was raised to 110° C. for distillation, and 100.8 g of high-purity difluorophosphoric acid distillate was collected with a yield of 89%.
[0082] (2) In a nitrogen atmosphere, at -5 to 15°C, drop 100.8g of high-purity difluorophosphoric acid into a solution prepared by 37.6g of lithium methoxide and 212g of methanol (water content ≤ 10ppm); the system was heated to 30°C for 2 hours, Filtrate was obtained by filtration.
[0083] (3) Under the pressure of -0.1MPa,...
Embodiment 3
[0088] This example provides a method for simultaneously preparing high-purity difluorophosphoric acid and high-purity lithium difluorophosphate, which specifically includes the following steps:
[0089] (1) Nitrogen atmosphere, at 22°C, from the bottom at a rate of 600mL / min, into a 500mL three-necked flask filled with 200g of dichlorophosphoric acid, feed a 20% fluorine-nitrogen mixture with a mass fraction of fluorine gas, and after 350min, The system was purged with nitrogen; the reaction system was heated to 110°C for distillation, and 137.5 g of high-purity difluorophosphoric acid fractions were collected, with a yield of 91%;
[0090] (2) Nitrogen atmosphere, at -5 to 15°C, drop 137.5g of high-purity difluorophosphoric acid into a solution prepared by 89g of lithium isopropoxide and 291g of isopropanol (water content ≤ 10ppm); the system is heated to 30°C After reacting for 4h, the filtrate was obtained by filtration.
[0091] (3) Under the pressure of -0.1MPa, heat th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com