Process and intermediates for the synthesis of caspofungin
A technology for compounds and acid addition salts, applied in the field of preparing these intermediates, can solve problems such as not using known methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0172]
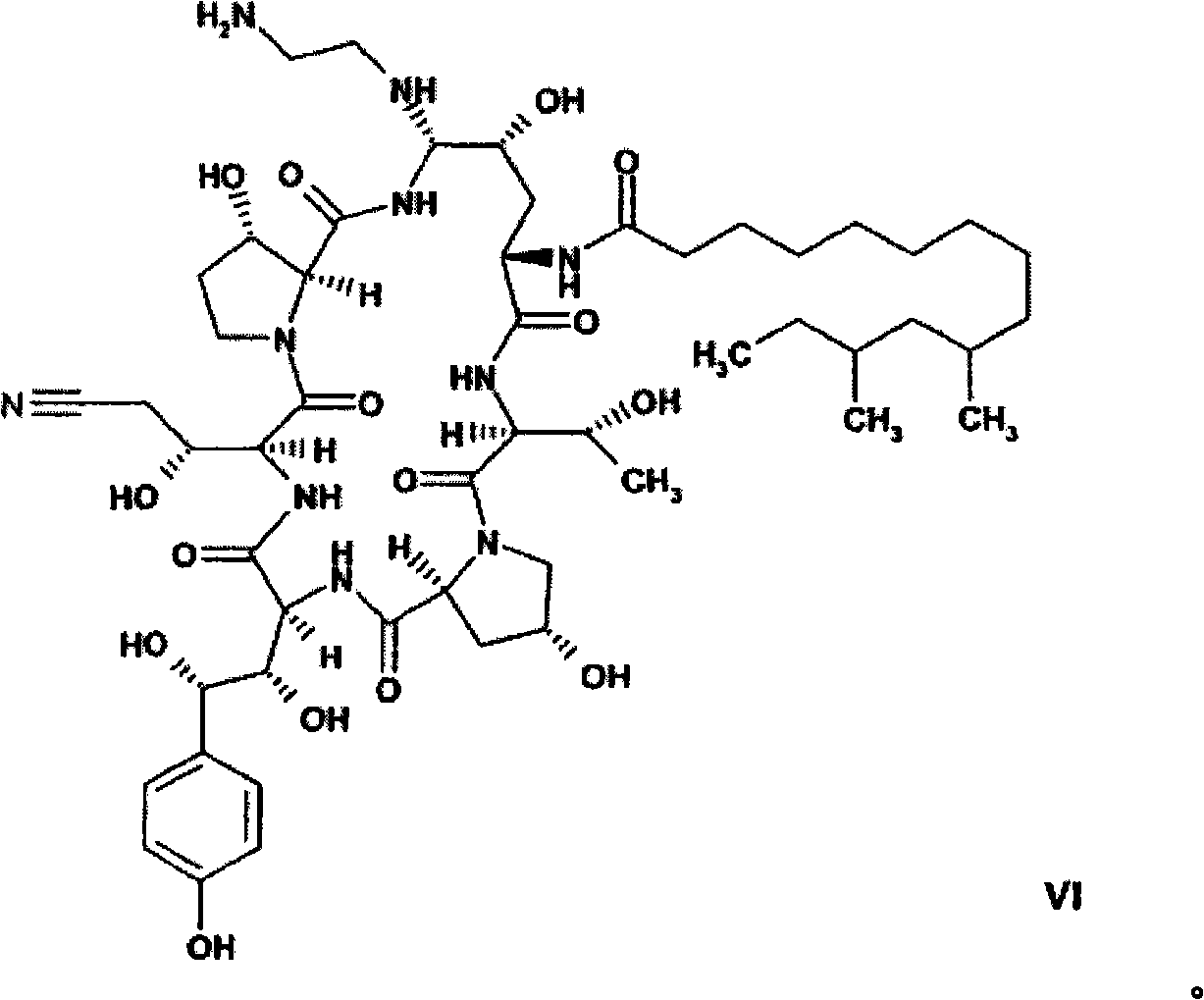
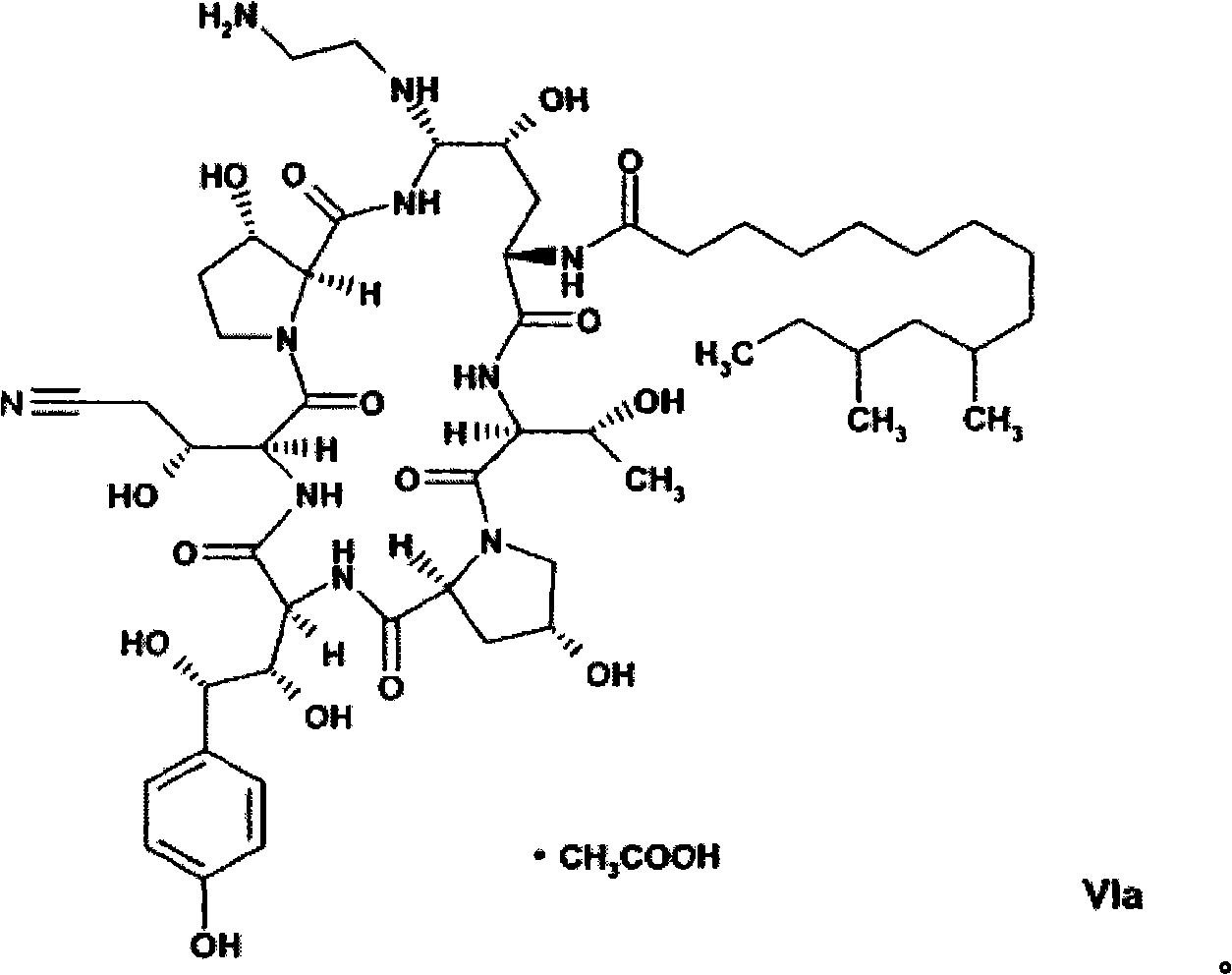
[0173] The compound of formula VI can be isolated from the reaction mixture by, for example, chromatography using RP-18 material followed by lyophilization of the enriched fraction. For chromatography, a mixture of acetic acid and acetonitrile may be used, wherein the ratio of acetic acid to acetonitrile is from about 60:40 to about 80:20, such as about 70:30, such as 75:25. The amorphous compound of formula VI can then be dissolved in an organic solvent, for example an alcohol such as methanol or ethanol, in the presence of acetic acid. By adding an anti-solvent, such as an ester, such as C of acetic acid 1 -C 4 Alkyl esters, such as ethyl acetate, can crystallize the monoacetate salt of the compound of formula VI. In addition, crystallization effectively removes impurities such as the C-35 epimer (homotyrosine moiety), which are difficult or impossible to remove economically by chromatography.
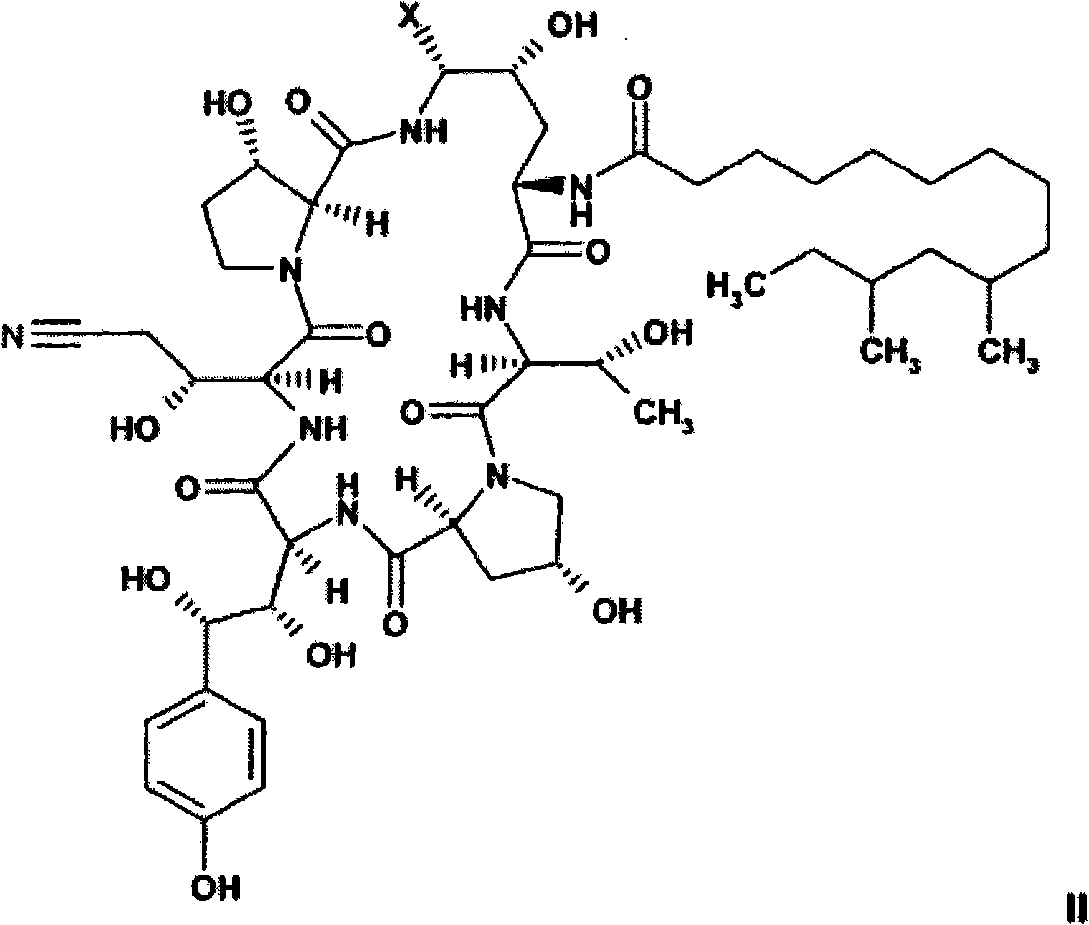
[0174] As described herein, compounds of formula II are intermedi...
Embodiment 1
[0191] Embodiment 1: the preparation of formula IV compound
[0192] Pneumocandin B 0 (10.2g) was dissolved in a mixture of dry 1-methyl-2-pyrrolidone (90ml) and dry N,N-dimethylformamide (10ml). The pale yellow solution was cooled to -20°C, and cyanuric chloride (4.2 g) was added in one portion. The mixture was stirred at -20°C until 98% conversion was reached (HPLC, ca. 3.5 hours). Water (100ml) was added over 10 minutes and the mixture was allowed to warm to ambient temperature.
[0193] The crude mixture was slowly poured into vigorously stirred water (1400ml). The suspension was aged for 2h, then filtered. The product was washed thoroughly with water and dried under vacuum. This material (9.3 g) was used in the next reaction without further purification.
Embodiment 2
[0194] Embodiment 2: the preparation of formula IV compound
[0195] Pneumocandin B 0 (10.0g) was dissolved in dry N,N-dimethylformamide (100ml). The water content of the solution was measured and adjusted to about 0.15%. The solution was cooled to -20°C, and cyanuric chloride (4.2 g) was added in one portion. The mixture was stirred at -20°C until 97% conversion was achieved (HPLC, ca. 1.0 hr). Water (100ml) was added over 10 minutes and the mixture was allowed to warm to ambient temperature.
[0196] The crude mixture was slowly poured into vigorously stirred water (1400ml). The suspension was aged for 2h, then filtered. The product was washed thoroughly with water and dried under vacuum. This material (8.2 g) was used in the next reaction without further purification.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com