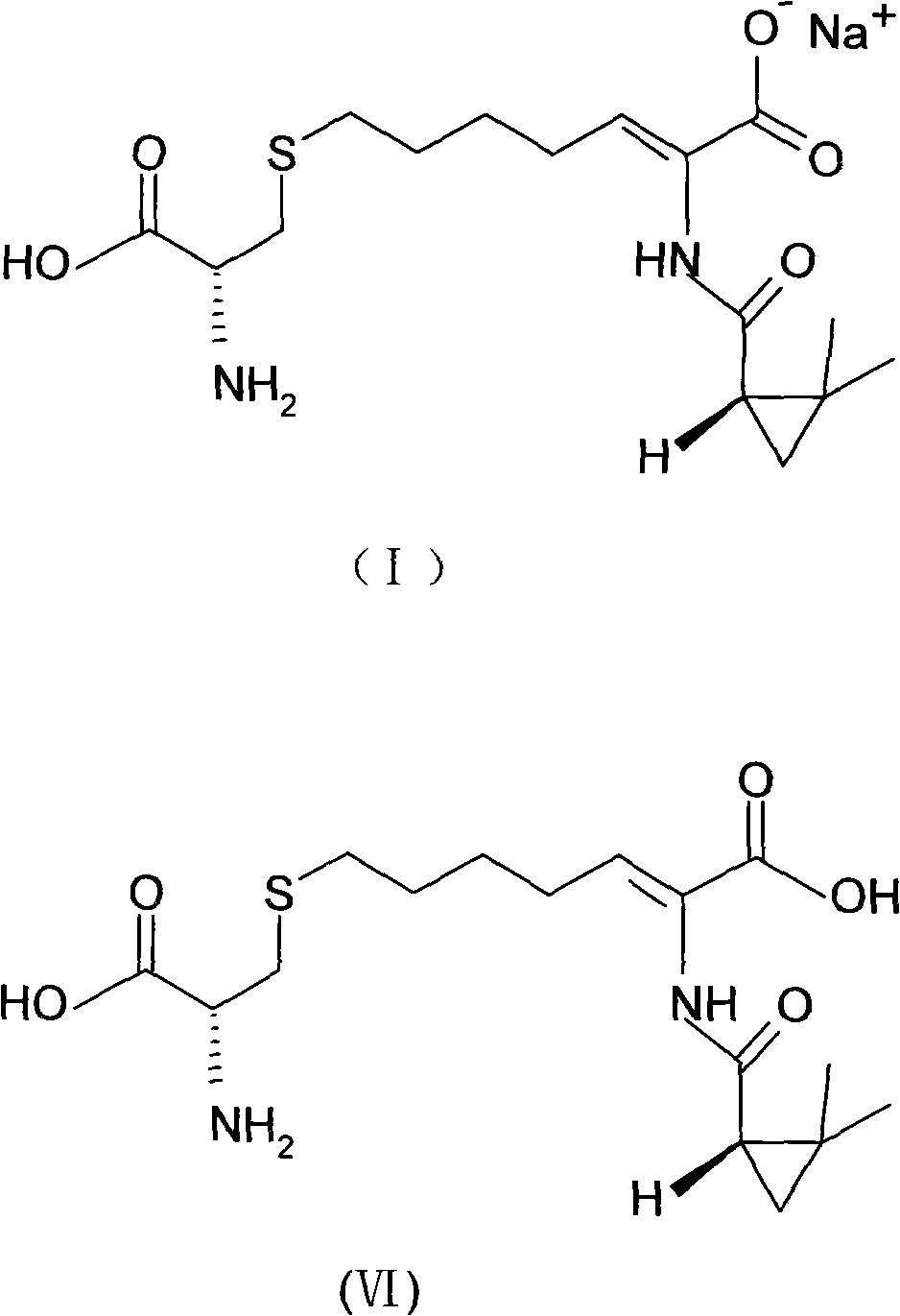
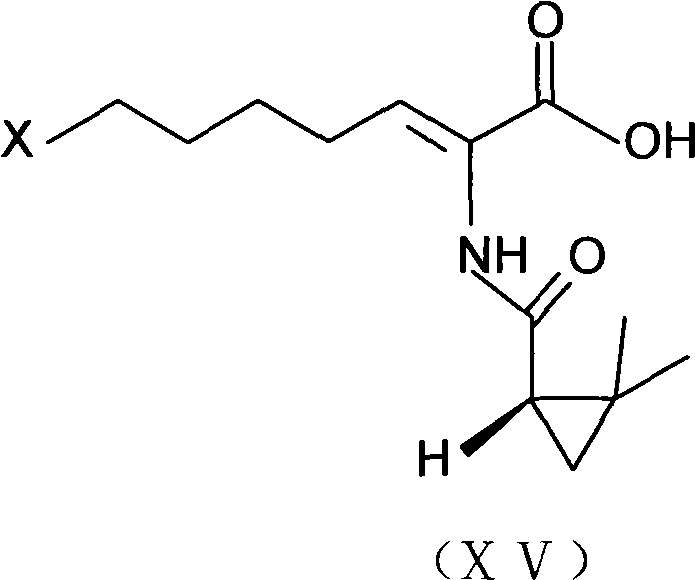
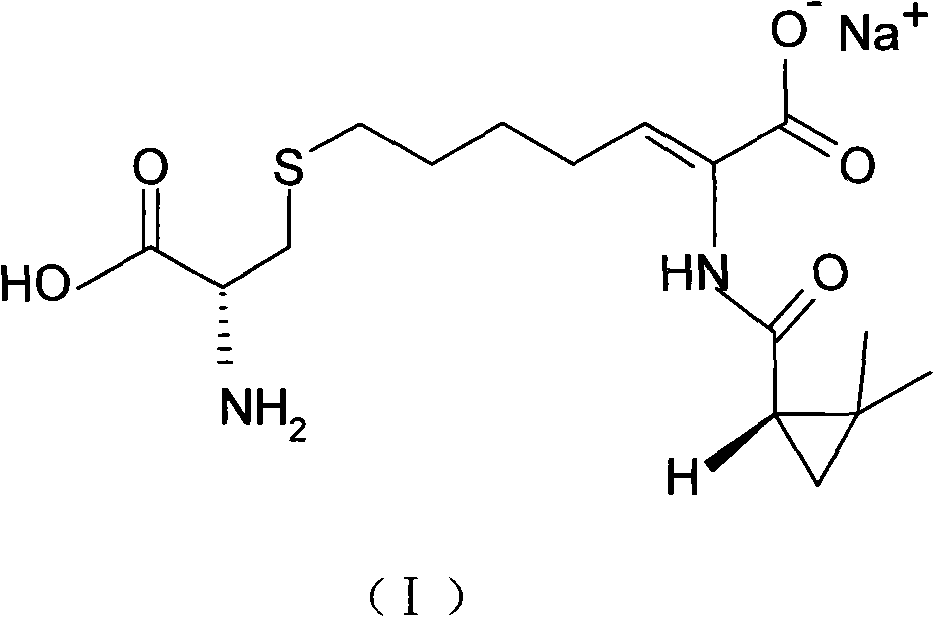
Process for preparing cilastatin sodium
A technology of cilastatin and sodium heptenoate, applied in the directions of sulfide preparation, chemical instruments and methods, preparation of organic compounds, etc., can solve the problem of not industrialized production of cilastatin sodium, reducing product yield and purity, Failure to meet economic requirements, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0037] The present invention is further described by the following examples to make the present invention clearer and easier to understand.
[0038] Step 1 Preparation of (Z)-7-chloro-2((S)-2,2-dimethylcyclopropylcarboxamido)-2-heptenoic acid (IV)
[0039] (Z)-7-chloro-2((S)-2,2-dimethylcyclopropylcarboxamido)-2-heptenoic acid (IV) was used according to the method reported in EP 48301B1 with 247.8g 7-chloro- Ethyl 2-oxoheptanoate (III), 135.6g (+)-2,2-dimethylcyclopropanecarboxamide and 1.6g p-toluenesulfonic acid were synthesized in 1200mL toluene, concentrated and reclaimed toluene to give 359.3 g brown viscous liquid, the product was directly used in the next reaction without separation.
[0040] Add 359.3g of the brown viscous liquid obtained in the previous step into 600mL of ethanol and 720g of 10% sodium hydroxide solution, heat to 45-50°C, heat and stir to make it react, monitor the reaction process by HPLC, and the reaction is completed in about 10 hours; then Add t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com