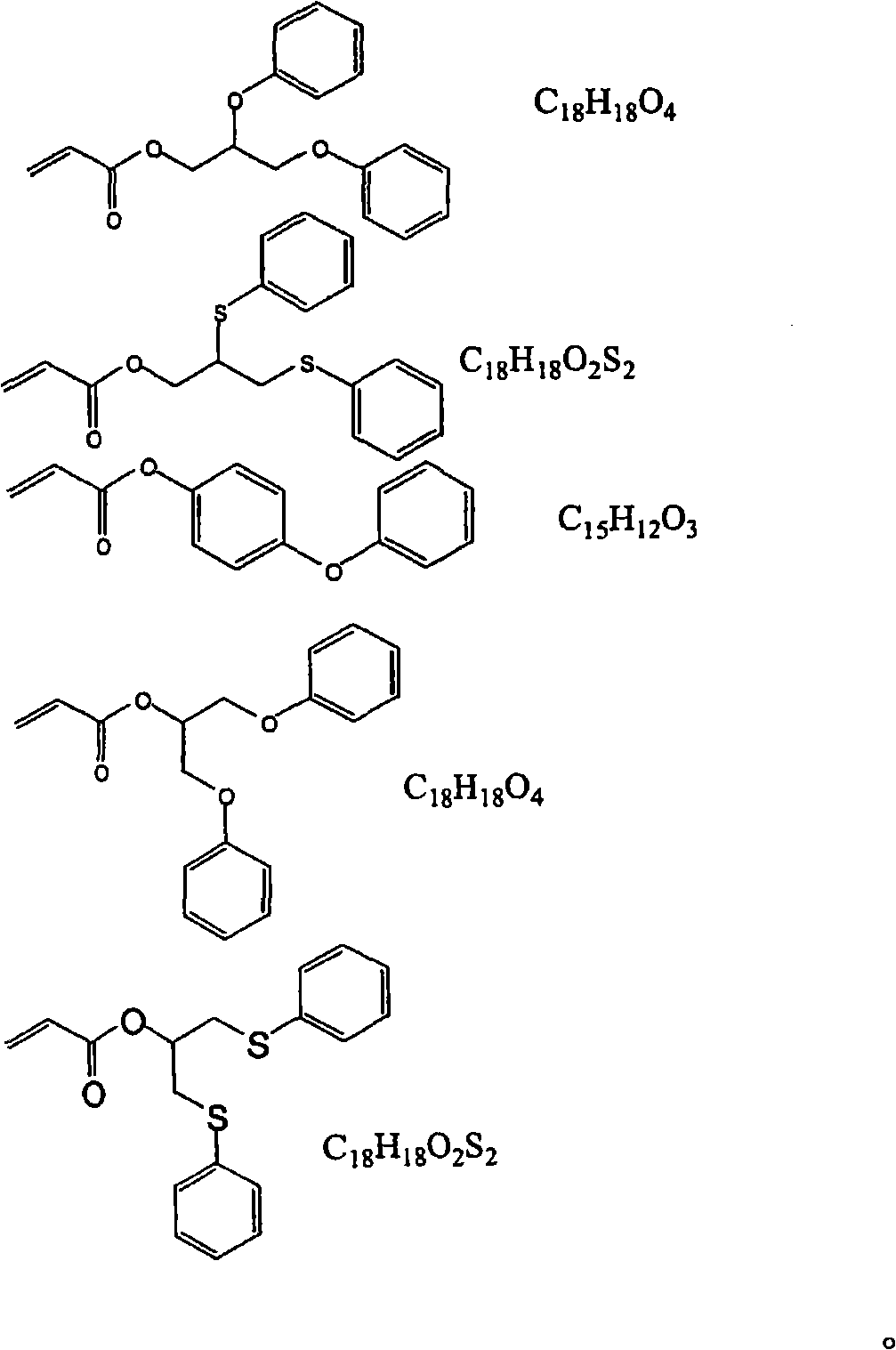
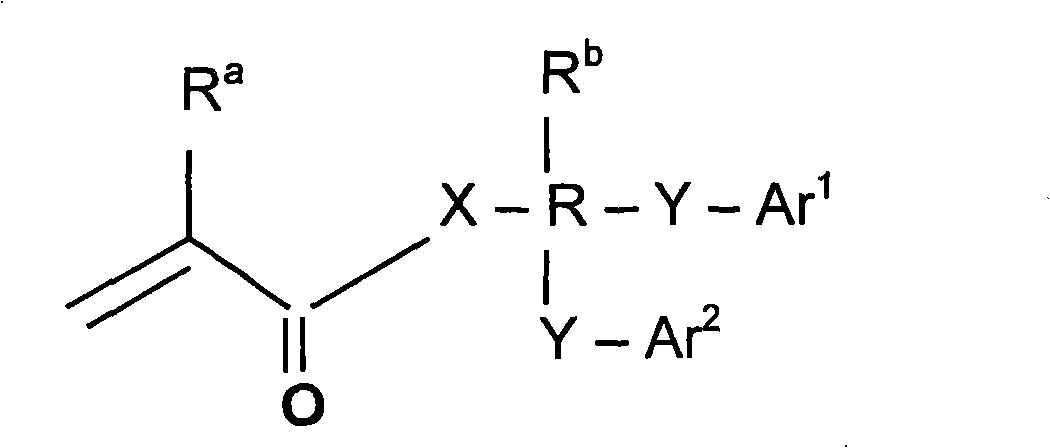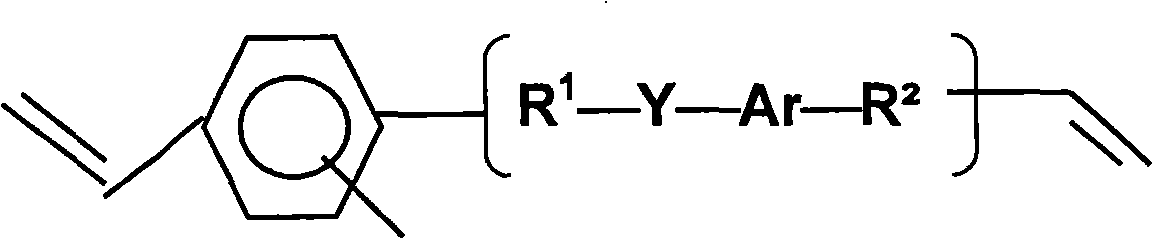Polymer composition having a high refractive index
A technology for polymerizing compositions and compounds, used in tissue regeneration, medical science, prostheses, etc., can solve the problems of unsatisfactory materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] synthesis
[0078]
[0079] (The above Mol.gew. refers to the molecular weight)
[0080] Reaction equation
[0081]
[0082] (The above Mol.gew. refers to the molecular weight)
[0083] Reagent:
[0084] Methacryloyl chloride
[0085]
[0086] Methyl magnesium chloride
[0087] Experimental part
[0088] (2-Methacrylic acid-2-phenylthio-1-phenylthiomethyl-ethyl ester)
[0089] 1,3-Diphenylthio-propan-2-ol was reacted with methylmagnesium chloride and methacrylic acid chloride in equimolar proportions (ratio 0.03618 mol, respectively).
[0090] 1,3-Diphenylthio-propan-2-ol (MW=276.42): (10 g=0.03618 mol) was dissolved in THF previously distilled over Na / K. To this solution was added 0.03618 mol of methylmagnesium chloride [Acros, 22% by weight solution in THF] (MW=74.79)=(2.71 g (corresponding to 12.32 g of a 22% by weight solution)). This solution was then slowly added to the following solution via a dropping funnel: 0.03618 mol of methacryloyl chlor...
Embodiment 2
[0092] synthesis
[0093]
[0094] (The above Mol.gew. refers to the molecular weight)
[0095] Reaction equation
[0096]
[0097] (The above Mol.gew. refers to the molecular weight)
[0098] Chemicals
[0099] Methacryloyl chloride (as above)
[0100] Experimental part
[0101] 1,3-Diphenylthio-propan-2-ol was reacted with methacryloyl chloride. Place the following reagents in a preheated container with a condenser and N 2 Inlet 250 ml three-necked spherical flask: 0.03 mol of methacryloyl chloride (3.88 g, 97%), about 60 ml of pre-distilled THF without inhibitors as solvent. 8.29 g of 1,3-diphenylthio-propan-2-ol (0.030 mol) and 2.94 g of triethylamine (0.030 mol) (99% by weight) were added to the dropping funnel. The solution from the dropping funnel was slowly dropped into the three-necked flask. No cooling was required as the reaction was not exothermic. A white precipitate (NEt 3 ) HCl. Stirring was continued at room temperature for 1.5 hours, then the...
Embodiment 3
[0108] synthesis
[0109]
[0110] (The above Mol.gew. refers to the molecular weight)
[0111] Reaction equation
[0112]
[0113] (The above Mol.gew. refers to the molecular weight)
[0114] Reagent
[0115] Acryloyl chloride
[0116]
[0117] Experimental part
[0118] using a condenser and N 2 Inlet preheated three-neck flask, 0.03 mol of acryloyl chloride (2.828 g, 96 wt %) as described above and 50-100 ml of inhibitor-free THF (pre-distilled) were placed in the flask as solvent. 8.29 g of 1,3-diphenylthiopropan-2-ol = 0.030 mol and 0.030 mol of triethylamine [99%] (4.1 ml) were placed in a dropping funnel. Allow the solution to drip slowly from the dropping funnel. No cooling was required as the reaction was not exothermic. A white precipitate (NEt 3 ) HCl. Stirring was continued for a further 1.5 hours at room temperature, then the precipitate was filtered and the THF was removed on a rotary evaporator. A yellow viscous liquid was obtained with a yie...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Refractive index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com