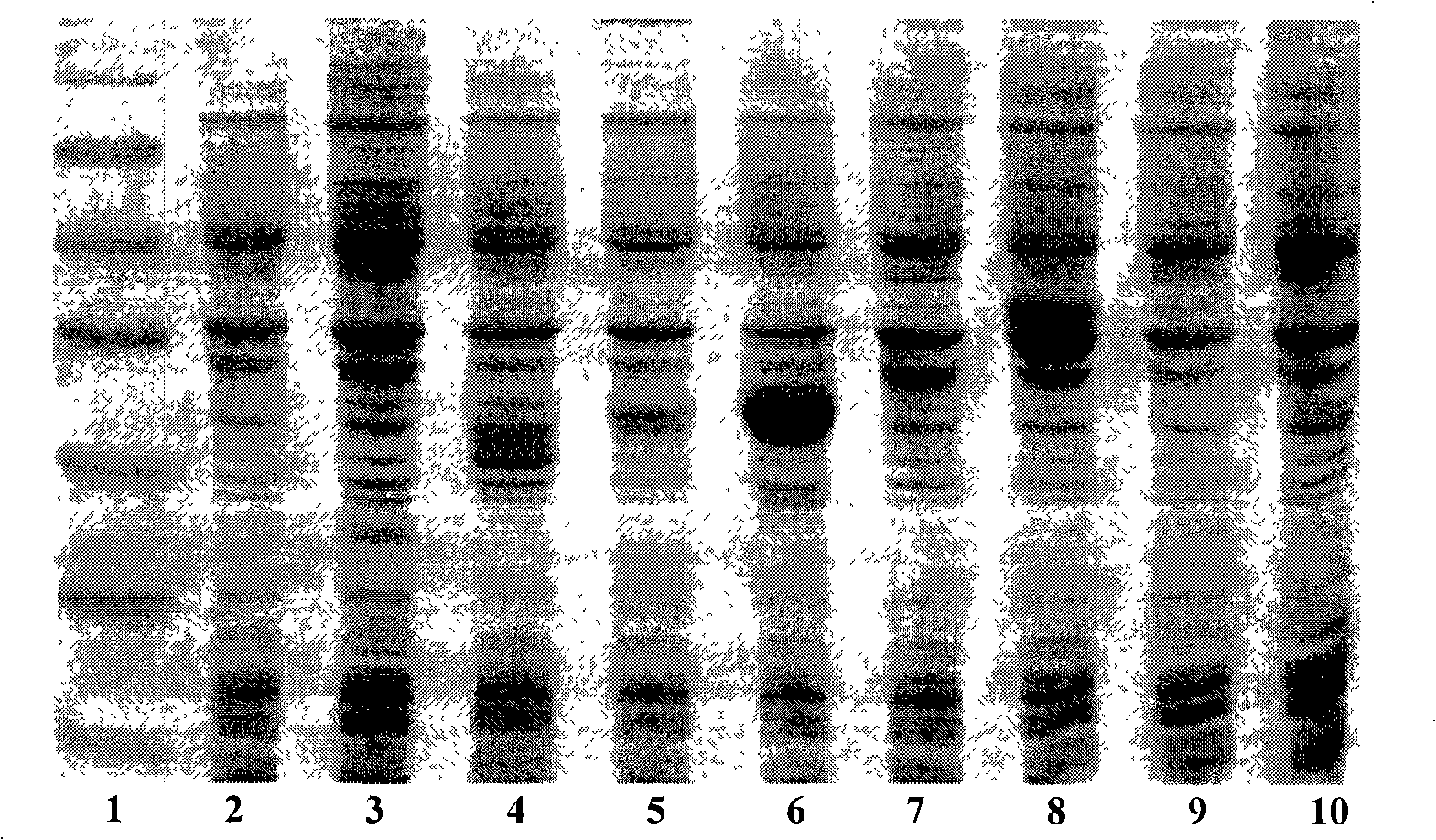Method for recombination, amalgamation and expression of series antimicrobial peptide gene
A fusion expression, antimicrobial peptide technology, applied in recombinant DNA technology, DNA/RNA fragments, introduction of foreign genetic material using vectors, etc., can solve the problems of low proportion, low expression and low solubility, and achieve high expression The effect of promoting soluble expression and increasing expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0019] Construction of recombinant expression vector and identification of transformants
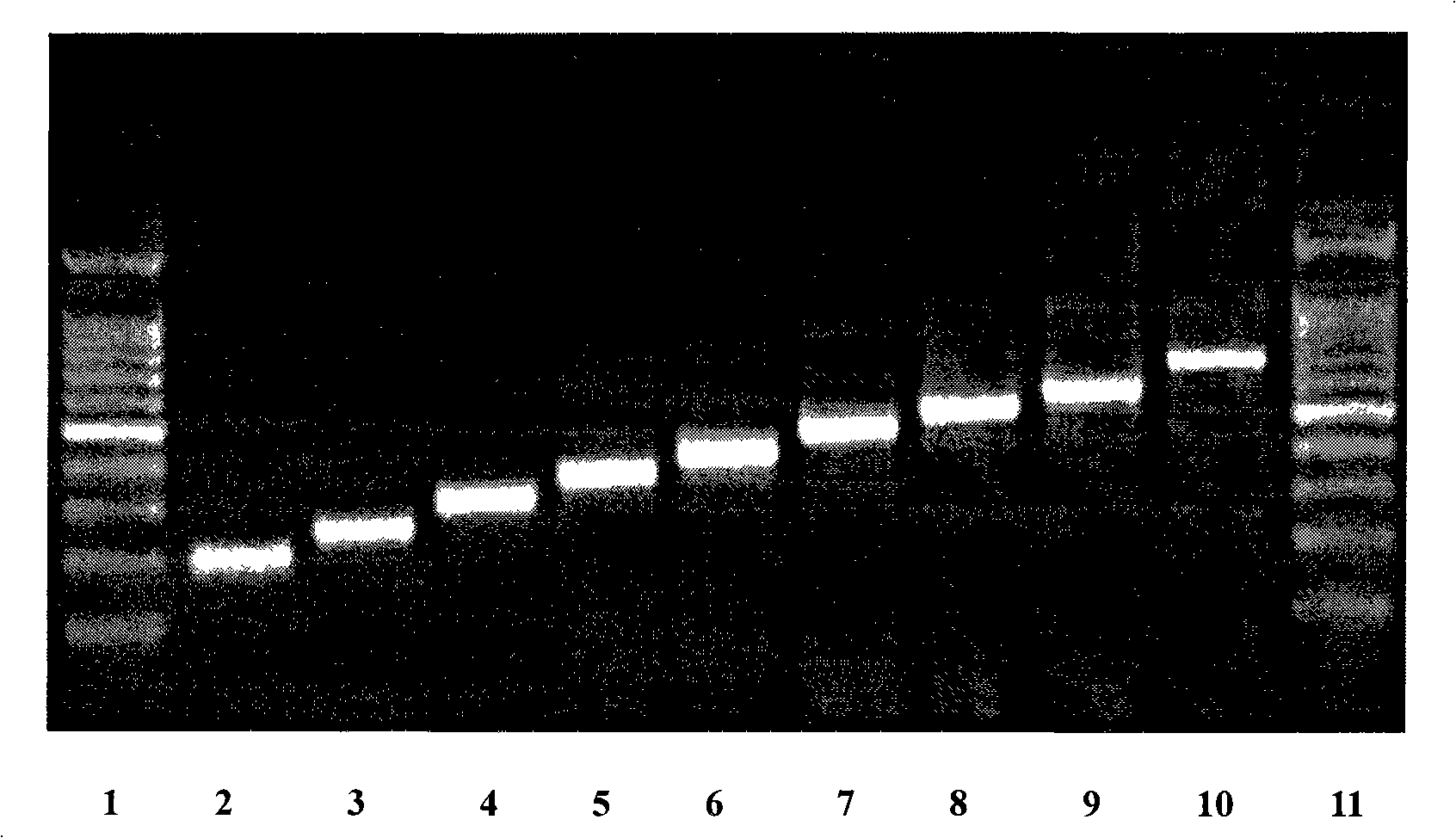
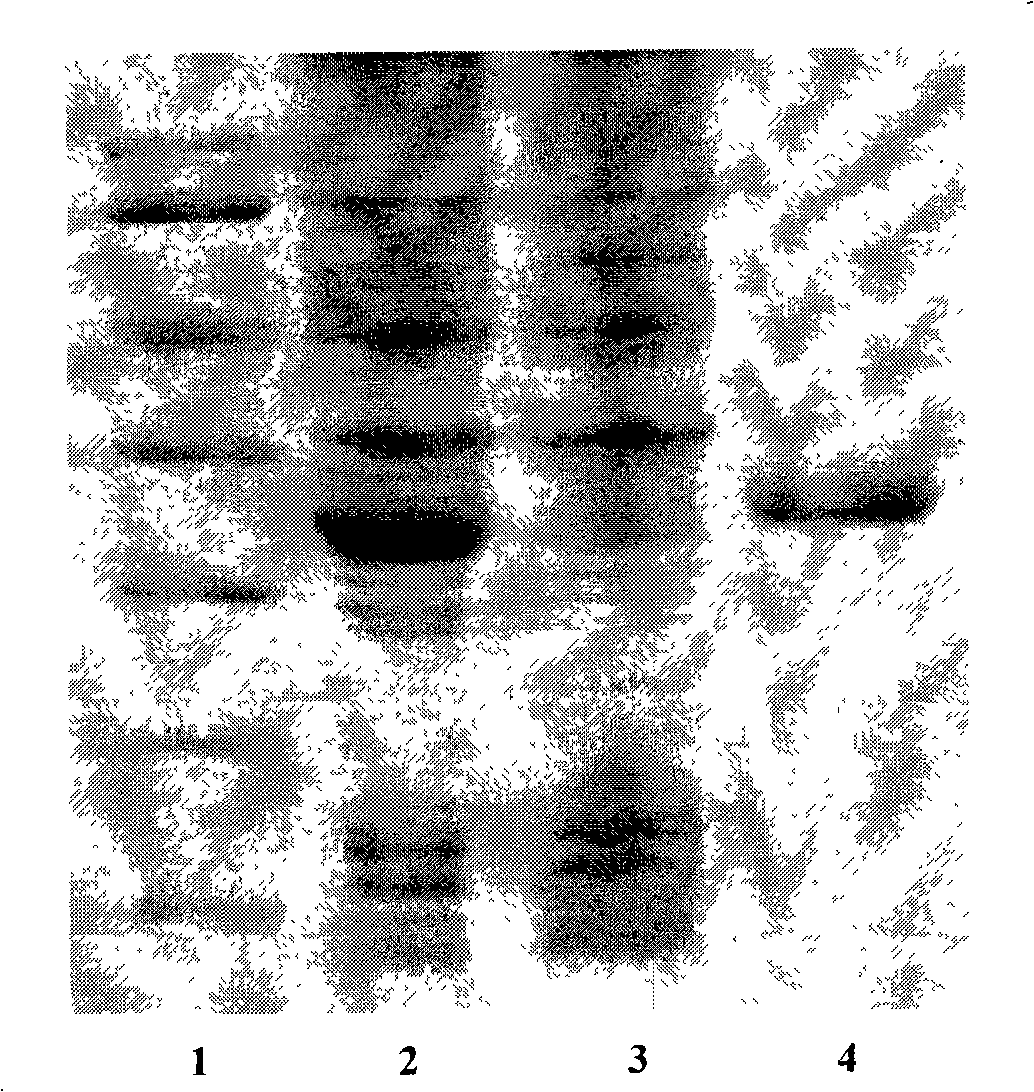
[0020] The nucleotide sequence of the gene was designed according to the codon preference of Escherichia coli, the sense and antisense strands were synthesized respectively, and a multi-copy gene was formed through the non-mirror symmetry complementary cohesive ends, and the pre-linker containing the BamH I restriction site and the Hind containing Post linker ligation of the III restriction site. The multi-copy gene with the linker was digested with HindIII and BamH I, and then ligated with the linearized pET32a vector to construct the recombinant plasmid expression vector pET32a-(LfcinB 15-W4, 10) n , After transforming Escherichia coli DH5α, positive recombinants containing different copy numbers of coding genes were obtained by colony PCR identification and screening according to the fragment size of the amplified product.
[0021] Figure 1 shows the results of agarose gel electropho...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com