Improved jasmonate derivatives, pharmaceutical compositions and methods of use thereof
A technology of compounds and hydrates, applied in the field of chemotherapeutic agents for the treatment of cancer, can solve problems such as the therapeutic application of derivatives that have not been described
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
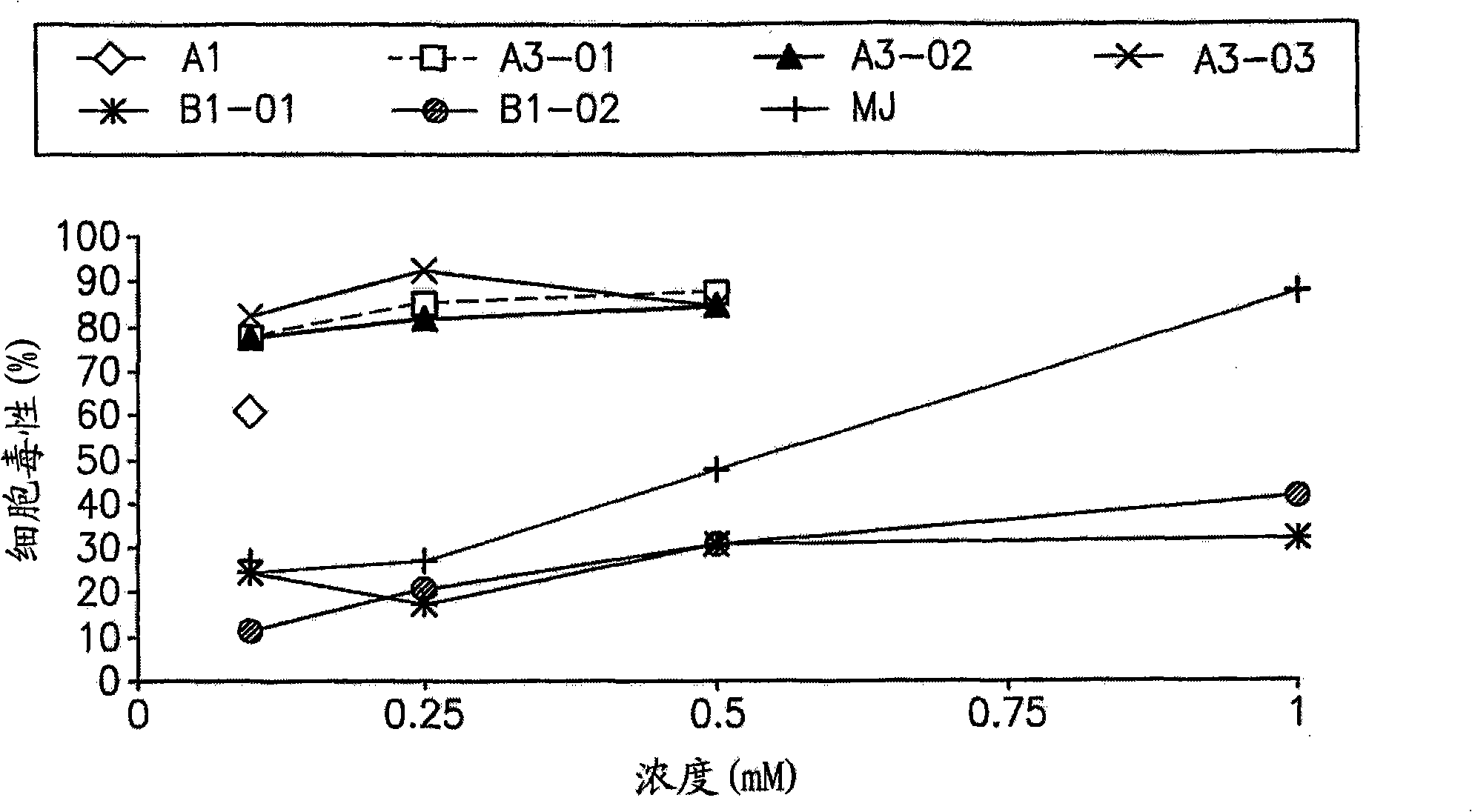
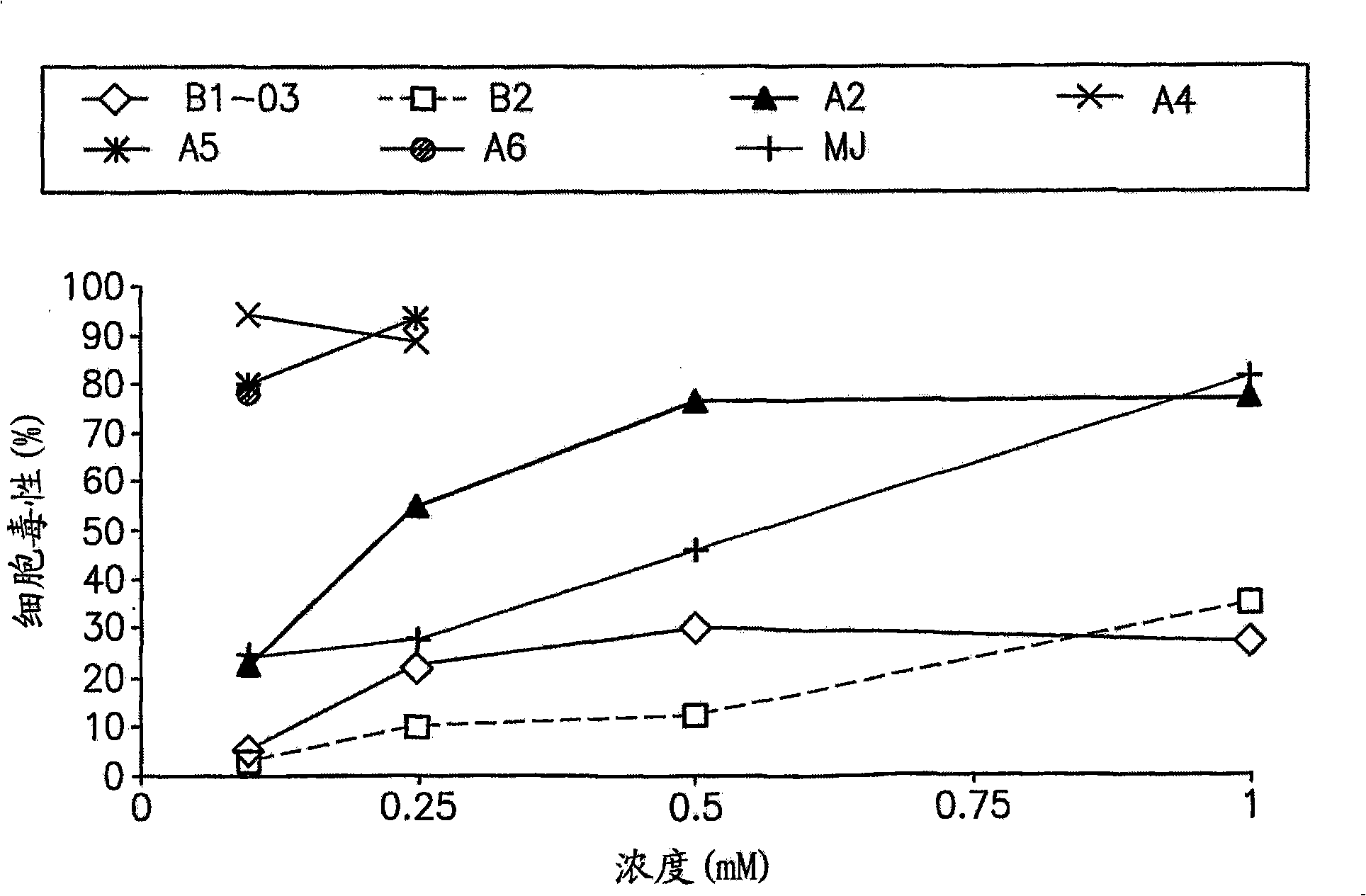
[0260] Example 1: Cellular Effects of Jasmonate Derivatives A1-A6, B1 and B2 on Leukemic Cells toxicity
[0261] The cytotoxicity of jasmonate derivatives A1-A6, B1 and B2 was compared with that of previously studied jasmonate, methyl jasmonate (MJ). Cytotoxicity of each compound was determined in Molt-4 (human acute lymphoblastic leukemia cell line).
[0262] experiment settings
[0263] Inoculate Molt-4 lymphoid leukemia cells (1.5x10 4 / well), and jasmonate derivatives in the concentration range of 0.1-1mM were added and cultured for 24 hours. Three parallels were performed for each experimental point. Untreated cells were used as controls. Test compounds were prepared as 100 mM stocks in dimethylsulfoxide (DMSO). Dilutions were made in medium and DMSO such that the maximum concentration of DMSO in each well was 0.5%. This DMSO concentration by itself did not affect the viability of any cell lines. Cytotoxicity and optical density were determined as described a...
Embodiment 2
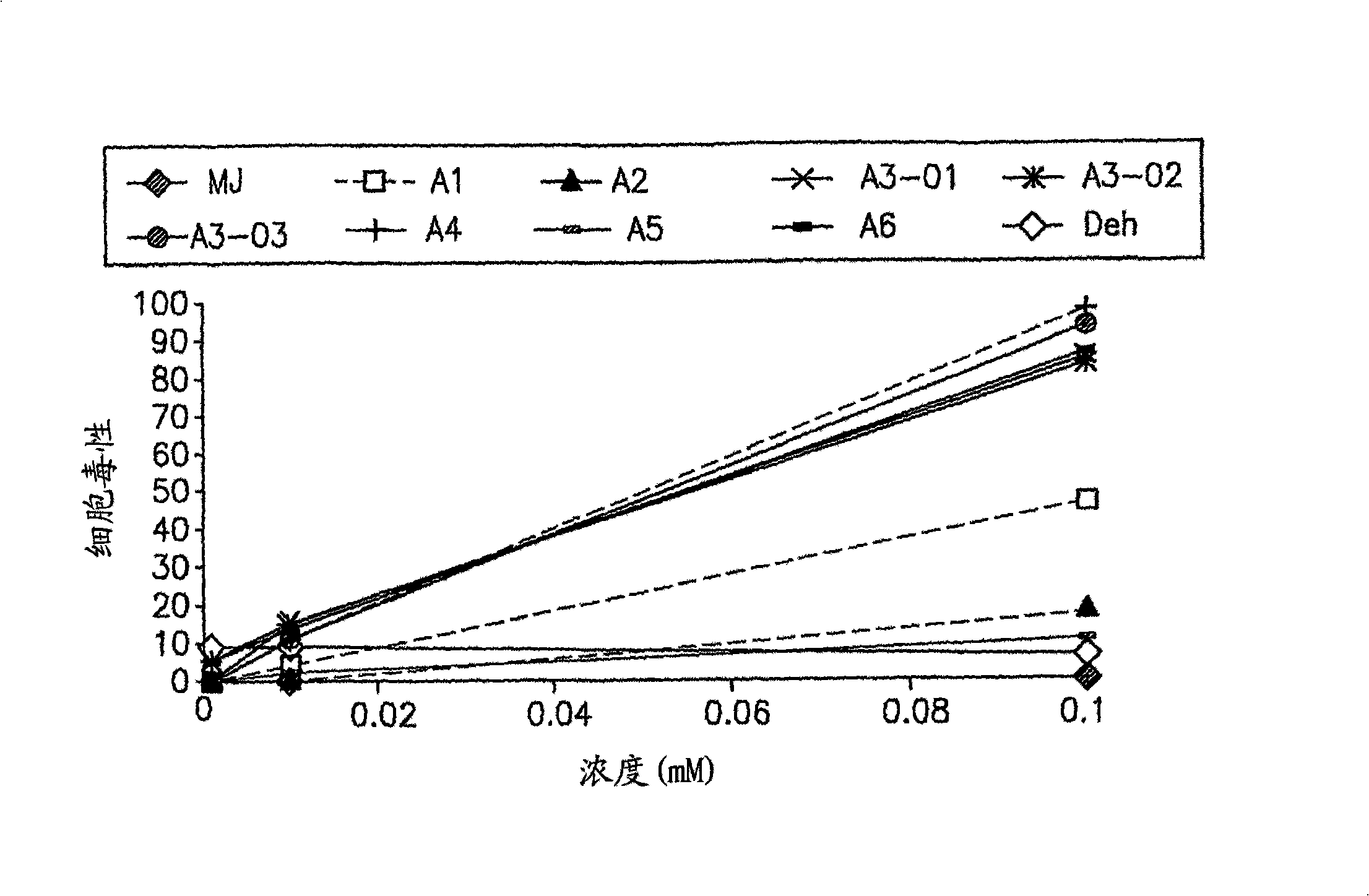
[0270] Example 2: Compounds A3 and A4 selectively fight cancer cells
[0271] Experimental setup:
[0272] Molt-4 ( leukemia) cells (2.5x10 4 / ml) and normal peripheral blood lymphocytes (PBL, 2x10 5 / ml) one day. In some experiments, PBLs were preincubated with 0.8 μg / ml phytohemagglutinin (PHA) and 5 ng / ml TPA for 48 or 24 hours to induce their entry into the cell cycle. These cell proliferations are similar to those of cancer cells, making the comparison more valid. The optical density of viable cells was determined as described above.
[0273] result
[0274] Below, shown in Table 2 below are IC 20 and IC 50 Level.
[0275] Table 2
[0276]
[0277] * ~20 repetitions
[0278] ** A3 and A4, two repetitions
[0279] According to previously reported data, MJ is cytotoxic to Molt-4 cells and almost non-toxic to normal lymphocytes. Compound A3 was found to be toxic to Molt-4 cells (IC 50 =0.05mM), while the toxicity to normal lymphocytes (-PHA / TPA) was ...
Embodiment 3
[0281] Example 3 - Cytotoxicity of oligomeric jasmonate derivatives on leukemia cells
[0282] The cytotoxicity of the novel MJ derivatives C1 and C2 was tested in 3 cancer cell lines:
[0283] A) Molt-4-human acute lymphoblastic leukemia cell line
[0284] B) CT26-murine colon cancer cell line
[0285] C) MCF7-human breast cancer cell line
[0286] The new derivatives were also tested in normal lymphocytes (PBL) obtained from healthy donors. The experimental setup is listed below along with the obtained ICs for the different cell lines 50 value.
[0287] Experimental setup:
[0288] Mononuclear cells were isolated from peripheral blood of healthy donors by ficoll-hypaque density gradient centrifugation. Monocytes were allowed to attach to plastic dishes to remove macrophages. The cell density is as follows: Molt-4 (2.5×10 in 100 μL per well 4 cells), CT26 (5×10 in 100 μL per well 3 cells), MCF7 (5×10 in 100 μL per well 3 cells) and PBL (1.5×10 in 100 μL per well...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com