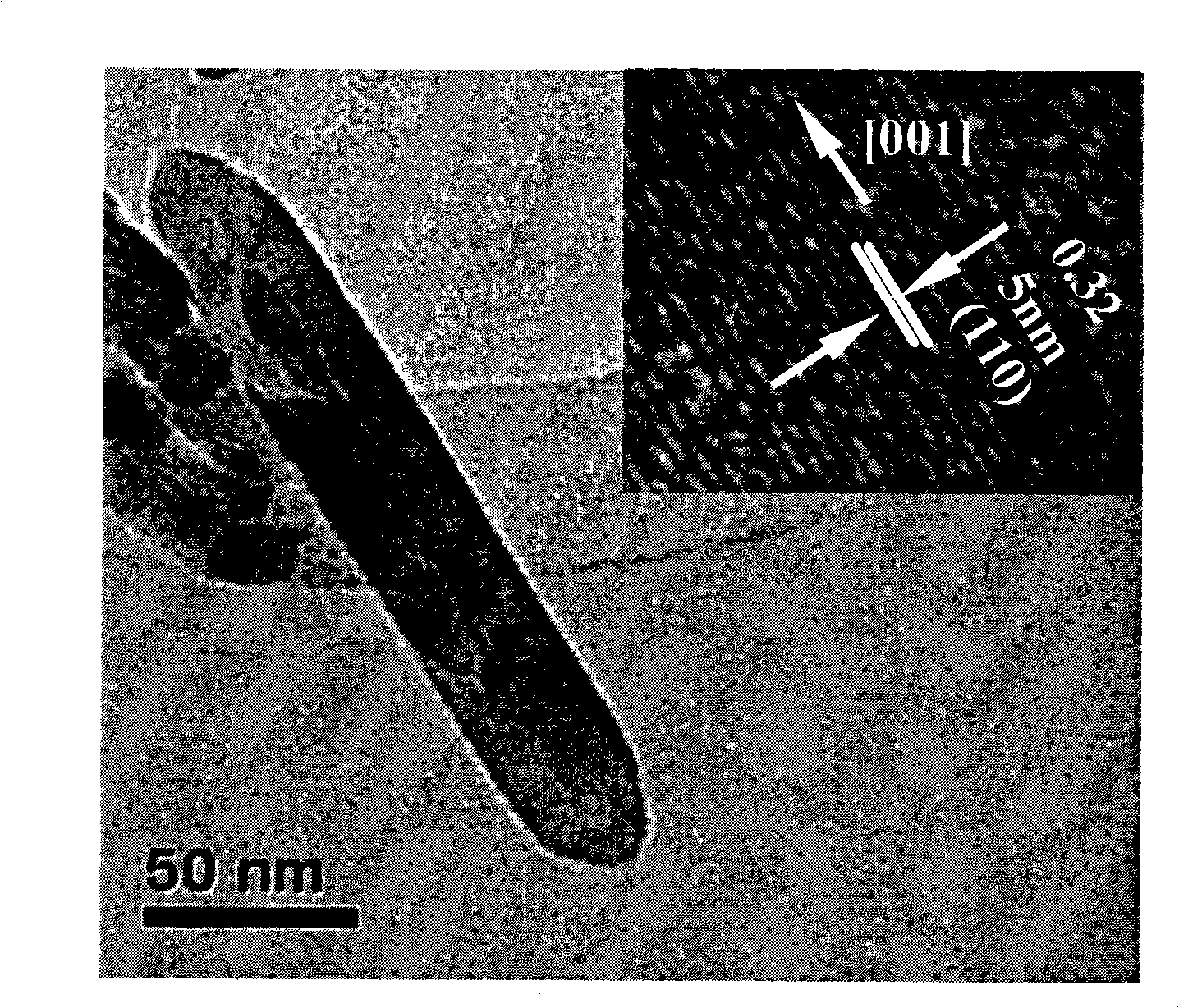
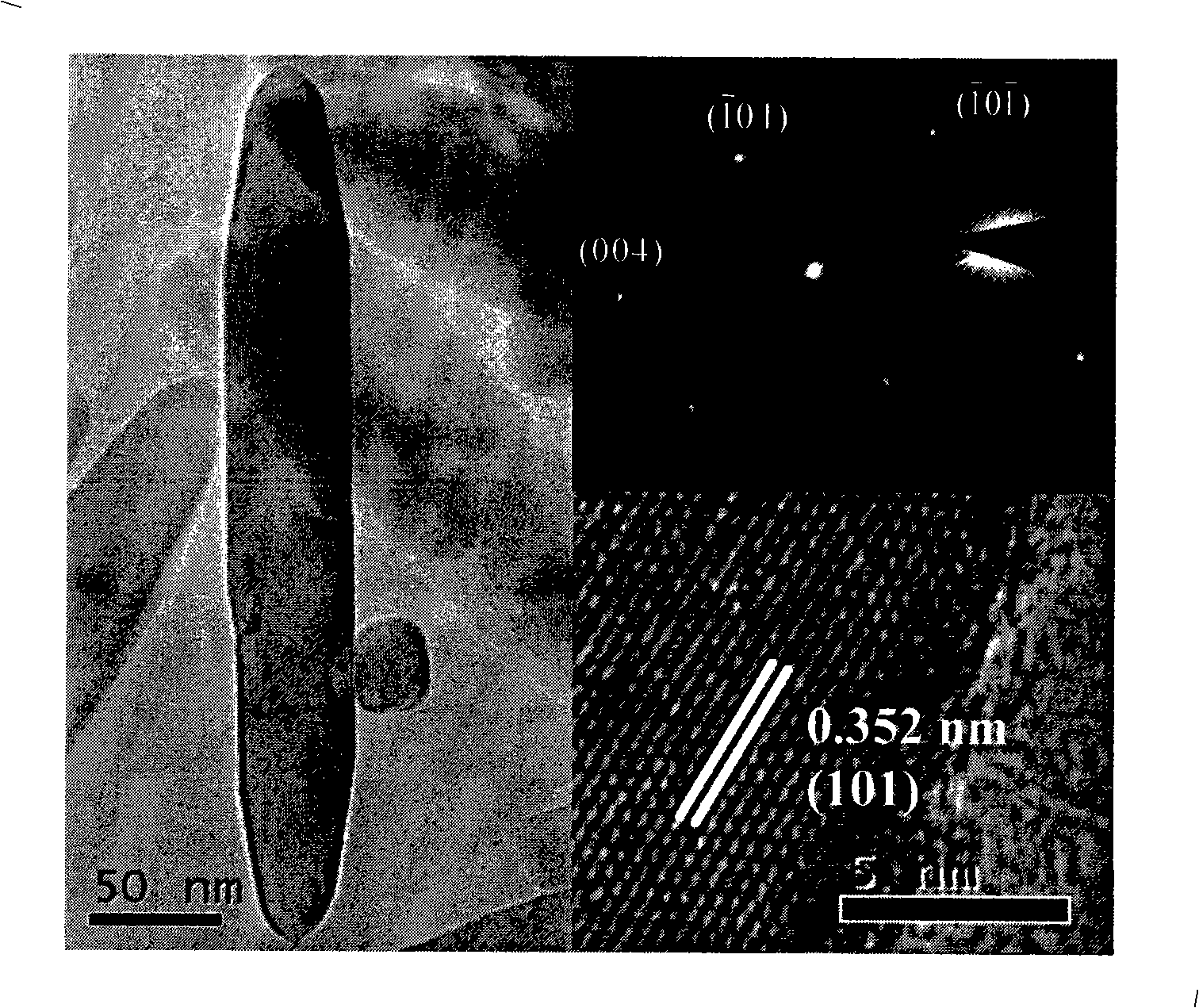
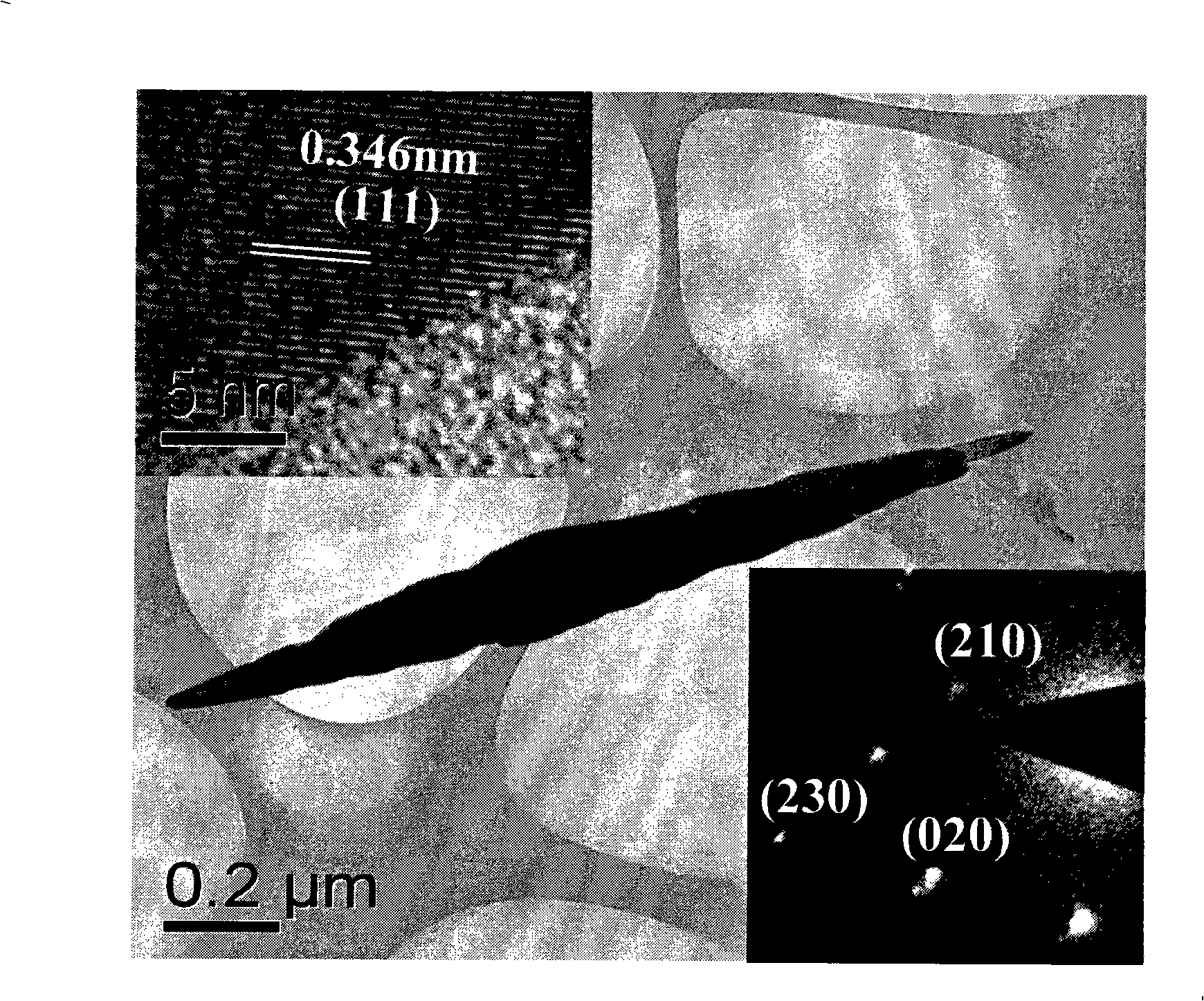
Method for controllable synthesis of pure phase anatase, red schorl, brookite titania nanorod
A technology of titanium dioxide and nanorods, applied in the field of nanomaterials, can solve the problems of insufficient purity of titanium dioxide nanomaterials, difficulty in synthesizing pure-phase brookite, complex preparation process, etc., and achieve low cost, easy operation, and scientific and reasonable preparation process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Controlled synthesis of phase-pure anatase titania nanorods
[0026] Mix 0.5g of titanium dioxide powder and 10mL of alkali solution with a concentration of 8 mol / L in a stainless steel autoclave or a polytetrafluoroethylene container, and react the mixture at 363K for 24 hours; use the resulting product as a precursor, and use the precursor Adjust the pH value to 5 and place it in a stainless steel autoclave or polytetrafluoroethylene container to react at 433K for 48h to obtain pure phase anatase titanium dioxide nanorods.
[0027] The alkali in the alkaline solution is caustic soda.
[0028] The pH value of the precursor is controlled by a pH meter, and the pH value is adjusted by caustic soda or nitric acid.
[0029] The synthesized anatase titania nanorods have a length of 100nm, a diameter of 20nm, and a purity of 99%.
Embodiment 2
[0031] Controlled synthesis of phase-pure anatase titania nanorods
[0032] 2g of titanium dioxide powder and 20mL of alkali solution with a concentration of 12 mol / liter are fully mixed in a stainless steel autoclave or a polytetrafluoroethylene container, and the mixture is reacted at 373K for 48h; the resulting product is used as a precursor, and the Adjust the pH value to 7, then place it in a stainless steel autoclave or a polytetrafluoroethylene container and react at 463K for 48h to obtain pure phase anatase titanium dioxide nanorods.
[0033] The alkali in the alkaline solution is caustic soda.
[0034] The pH value of the precursor is controlled by a pH meter, and the pH value is adjusted by caustic soda or nitric acid.
[0035] The synthesized anatase titanium dioxide nanorod has a length of 150nm, a diameter of 30nm, and a purity of more than 99%.
Embodiment 3
[0037] Controlled synthesis of phase-pure anatase titania nanorods
[0038] Mix 1-1.5g of titanium dioxide powder and 30mL of alkali solution with a concentration of 10 mol / L in a stainless steel autoclave or a polytetrafluoroethylene container, and react the mixture at 370K for 30-40h; use the resulting product as a precursor , the pH value of the precursor was adjusted to 6, and then it was placed in a stainless steel autoclave or a polytetrafluoroethylene container and reacted at 450-460K for 48 hours to obtain a pure phase anatase titanium dioxide nanorod.
[0039] The alkali in the alkaline solution is caustic soda.
[0040]The pH value of the precursor is controlled by a pH meter, and the pH value is adjusted by caustic soda or nitric acid.
[0041] The synthesized anatase titanium dioxide nanorod has a length of 150nm, a diameter of 30nm, and a purity of more than 99%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com