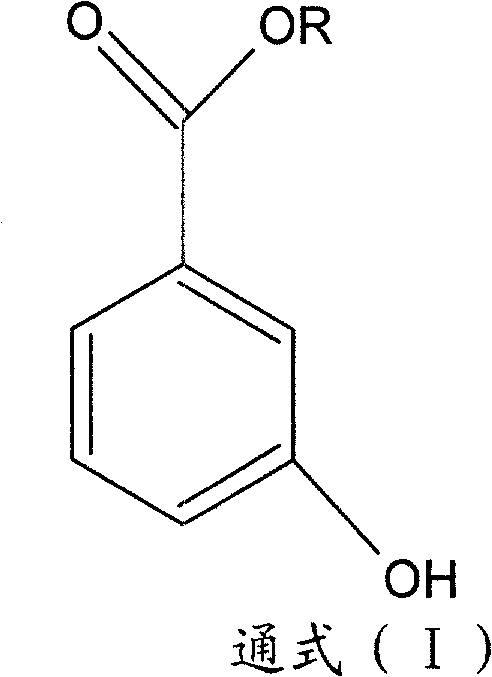
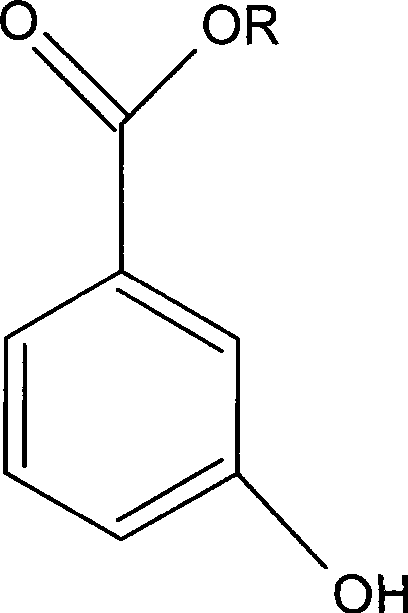
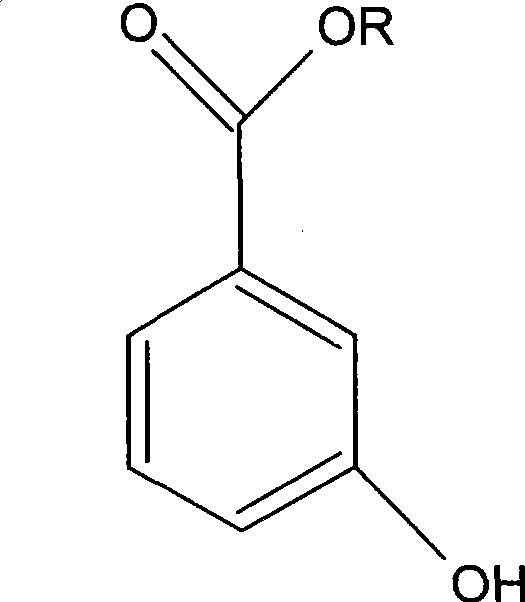
3-hydroxy benzoate preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Drop into 2kg of 3-hydroxybenzoic acid (purity ≥ 99.5%) in the reactor, add water 3000ml, stir to make the material in the reactor be slurry, control the internal temperature of the reaction system between 25 ℃ ~ 35 ℃, add dropwise 900 grams (purity ≥ 99.5%) 90%) KOH dissolved in 2500g water lye, until the reaction system PH = 6.4 ~ 6.5, add 60g of activated carbon to the system, stir at room temperature for 30 minutes, decarbonize and filter, spray dry the mother liquor, the inlet temperature is 150°C ~ 190°C, the outlet temperature 70°C-90°C, after spray drying, 2336g of dry solid was obtained, and the molar yield was 91.5%. Product testing results: moisture <1.0%, purity 99.73%.
Embodiment 2
[0033] Add 2 kg of 3-hydroxybenzoic acid (purity ≥ 99.5%) into the reactor, add 1500 ml of water and stir to make the material in the reactor slurry, control the internal temperature at 50°C to 65°C, and add 900g (purity ≥90%) of KOH solution dropwise. Add lye in 1500ml of water until the pH of the reaction system is 6.4~6.5, add 60g of activated carbon to the system, keep the internal temperature at 50℃~65℃, stir and decolorize for 30 minutes, decarbonize and filter, spray dry the mother liquor, and the inlet temperature is 170℃~180℃ , The outlet temperature is 75°C to 85°C. (in the present embodiment: acid: water=1:1.5, because the consumption of water is minimum, the 3-hydroxybenzoate aqueous solution concentration is higher. During spray drying: when the spout of atomizer is moderate in material spray velocity, can not Blockage; if the spray flow rate of the material is adjusted to the highest, the nozzle of the atomizer will occasionally form tiny solid blocks; if the con...
Embodiment 3
[0035] Add 2 kg of 3-hydroxybenzoic acid (purity ≥ 99.5%) in the reactor, add 6000 ml of water and stir to make the material in the reactor slurry, control the internal temperature at 50 ° C to 65 ° C, and drop 860 g (purity ≥ 98%) to obtain Na 2 o 3 Dissolve lye in 4000ml of water until the pH of the salt-forming system is 6.4~6.5, add 70g of activated carbon to the system, keep the internal temperature at 50℃~60℃, stir and decolorize for 30 minutes, decarbonize and filter, spray dry the mother liquor, and the inlet temperature is 158℃~ 190℃, outlet temperature 80℃~95℃. After spray drying, 2110 g of dry solid was obtained, and the molar yield was 91%. Product testing results: moisture <1.0%, purity 99.75%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com