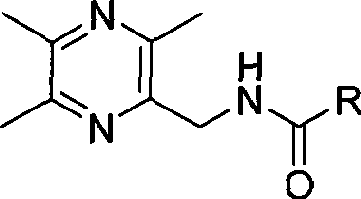
Tetramethylpyrazine acidamides derivates, preparation method and medicament composition and application
A technology of ligustrazine amide and derivatives, applied in the field of derivative drugs, can solve the problems of low bioavailability, fast metabolism, increased side effects and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
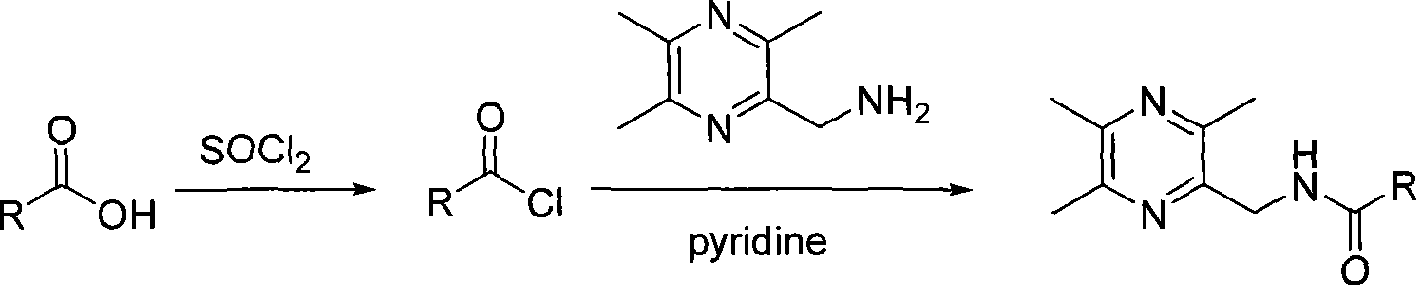
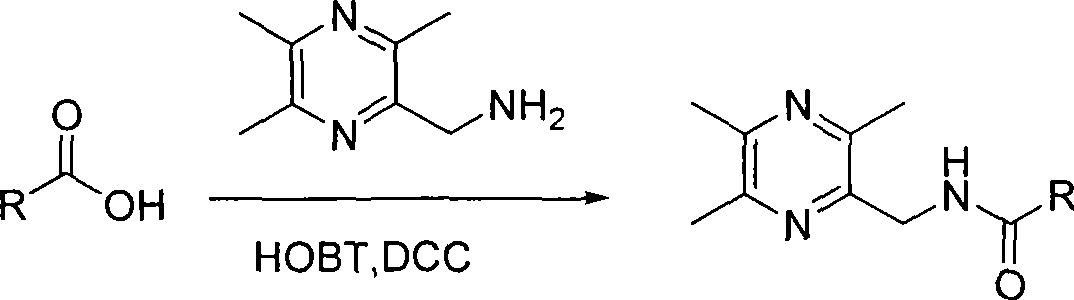
[0052] Embodiment 1: the preparation of 2-benzoyl-3,5,6-trimethylpyrazine (A1)
[0053] 0.755g, 0.005mol 2-methylamino-3,5,6 trimethylpyrazine and 0.4g, 0.0055mol pyridine were dissolved in about 30ml chloroform in a 150ml round bottom flask, benzoyl chloride (purchased pure product) 0.0775g, 0.0055mol was dissolved in about 30ml chloroform, and the latter solution was slowly dropped into the former solution in a constant pressure dropping funnel under the condition of an ice-water bath. Then the reaction solution was distilled under reduced pressure, and the obtained crude product was separated by a fast column (ethyl acetate:cyclohexane=4:1), and then recrystallized with 90-95% ethanol to obtain pure product 2-benzoyl-3,5 , 6-trimethylpyrazine (Al), white crystal, 0.66g, yield 52%, mp 115-116°C;
[0054] Spectral analysis data: IR(KBr, cm -1 ): 3279.86 (NH), 1652.95 (C=O), 1530.02, 1486.59, 1446.19, 1416.15 (C=N, C=C); 1 H-NMR (600MHz, CDCl 3 , δ ppm): 7.92 (s, 1H, NH), ...
Embodiment 2
[0055] Example 2: Preparation of 2-phenylacetyl-3,5,6-trimethylpyrazine (A2)
[0056] Prepared as described in Example 1, ethyl acetate:cyclohexane=4:1 fast column separation, yield 53%, yellow solid, mp 125-126°C;
[0057] Spectral analysis data: IR(KBr, cm -1 ): 3377.86 (NH), 1507.64, 1496.82, 1454.64 (C=N, C=C), 1662.40 (C=O); 1 H-NMR (600MHz, CDCl 3 , δ ppm): 7.17 (s, H, NH), 7.40-7.33 (m, 5H, Ar-H), 4.43 (d, 2H, CH 2 , J=4.1Hz), 2.46(s, 3H, CH 3 ), 2.42 (s, 3H, CH 3 ), 2.34 (s, 3H, CH 3 ); 13 C-NMR (150MHz, CDCl 3 , δ ppm): 171.13 (C=O), 144.57, 147.50, 147.73, 149.51 (pyrazine-C), 127.31, 128.97, 129.70, 134.91 (Benzene-C), 41.02, 43.84 (CH 2 ), 19.91, 21.21, 21.34 (CH 3 ); ESI-MS: 270.5 (M+H) + ;C 16 h 19 N 3 O.
Embodiment 3
[0058] Embodiment 3: Preparation of 2-m-chlorobenzamidomethyl-3,5,6-trimethylpyrazine (A3)
[0059] Prepared as described in Example 1, ethyl acetate:cyclohexane=4:1 fast column separation, yield 45%, white solid, mp 110-111°C;
[0060] Spectral analysis data: IR(KBr, cm -1 ): 3406.14 (NH), 1523.23, 1565.89, 1523.23 (C=N, C=C), 1671.43 (C=O); 1 H-NMR (600MHz, CDCl 3 , δ ppm): 7.98 (s, H, NH), 7.89 (t, H, Ar-H), 7.76 (dd, 1H, Ar-H, J 1 =1.11Hz,J 2 =7.73Hz), 7.50(m, 1H, Ar-H), 7.40(m, 1H, Ar-H), 4.67(d, 2H, CH 2 , J=4.51Hz), 2.54(s, 3H, CH 3 ), 2.54 (s, 3H, CH 3 ), 2.53 (s, 3H, CH 3 ); 13 C-NMR (150MHz, CDCl 3 , δ ppm): 165.99 (C=O), 144.61, 147.84, 148.00, 149.96 (pyrazine-C), 125.09, 127.55, 129.93, 133.14, 134.79, 136.15 (Benzene-C), 41.41 (CH 2 ), 20.00, 21.40, 21.49 (CH 3 ); ESI-MS: 290.5 (M+H) + ;C 15 h 16 ClN 3 O.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com