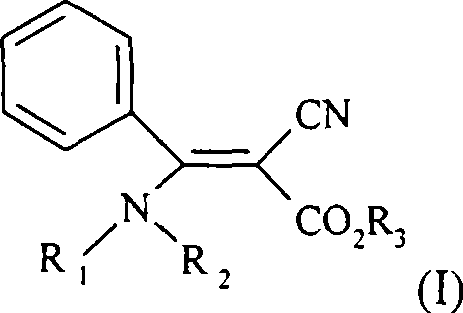
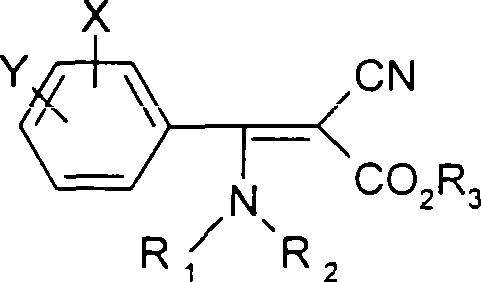
2-cyano-3-(substituted) amidine-3-phenyl acrylates compounds, preparation method and use thereof
A technology of phenyl acrylates and compounds, applied in the preparation of organic compounds, botany equipment and methods, chemical instruments and methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example
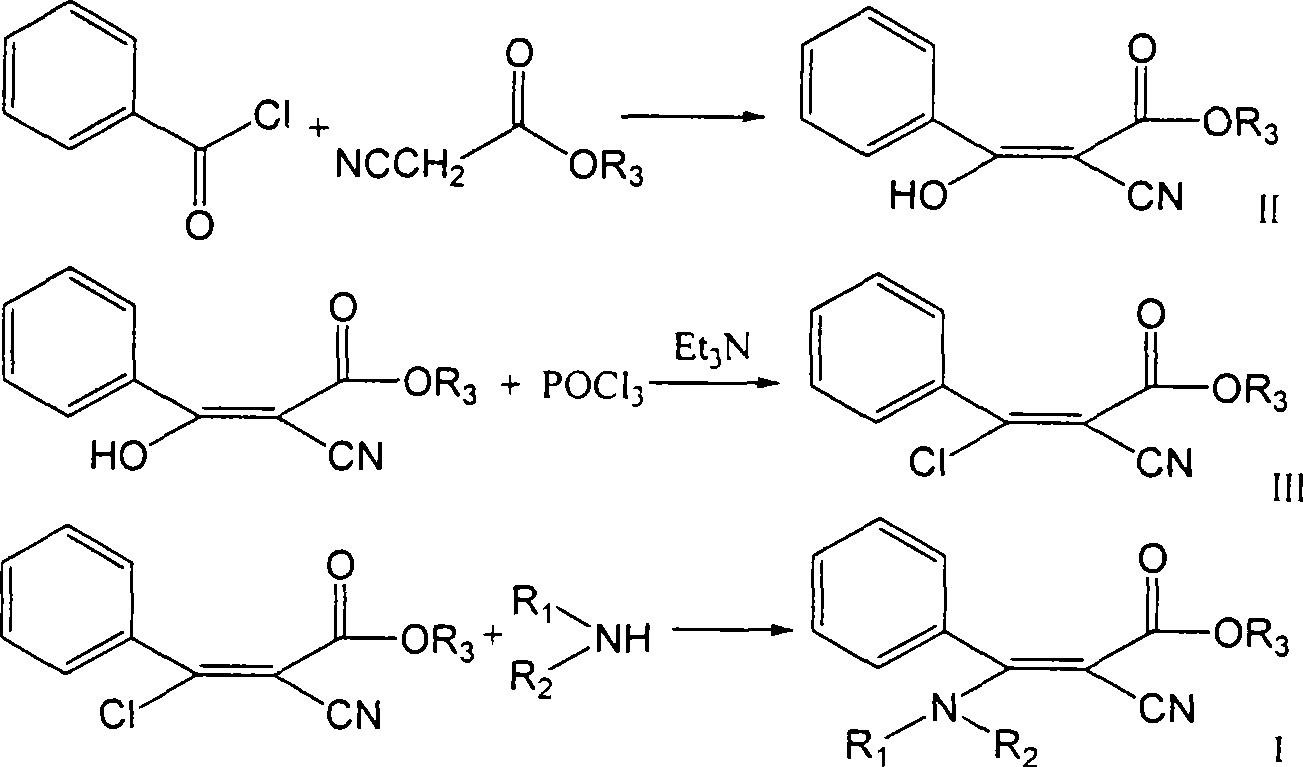
[0066] Synthetic example: 1 Compound No 1 Preparation of 2-cyano-3-(substituted) amino-3-amino-3-phenylacrylate n-propyl: (a) 2-cyano-3-hydroxyl-3-benzene The preparation method of n-propyl propionate
[0067] a-1: Preparation method 1 of n-propyl 2-cyano-3-hydroxy-3-phenylpropionate:
[0068] 24.3 grams of magnesium (1.0 moles) was placed in a 2-liter three-necked flask containing 500 milliliters of absolute ethanol and 500 milliliters of ethylene glycol dimethyl ether and 2 grams of carbon tetrabromide, stirred and reacted for 12 hours at 90° C., and depressurized After distilling ethanol and ethylene glycol dimethyl ether, add 500 milliliters of ethylene glycol dimethyl ether, stir and add 127 grams of n-propyl cyanoacetate (1.0 mole) dropwise below 20 ° C, the solution is cooled to 0 ° C, dropwise Add 140.5 g of benzoyl chloride (1.0 mol), and after the drop, the reaction solution was stirred and reacted at room temperature for 15 hours. The organic layer was washed with...
Embodiment 1
[0085] Formulation example 1: 10% EC
[0086] Compound No.1 10 parts
[0087] Tween 80 12 servings
[0088] N,N-Dimethylformamide 3 parts
[0089] Xylene make up 100 parts
Embodiment 2
[0090] Formulation Example 2: 90% Soluble Liquid
[0091] Compound No.1 90 parts
[0092] 10 parts of N-methylpyrrolidone
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com