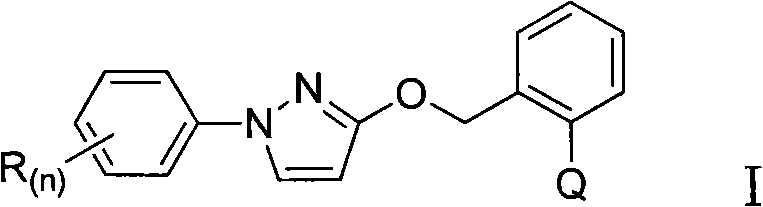
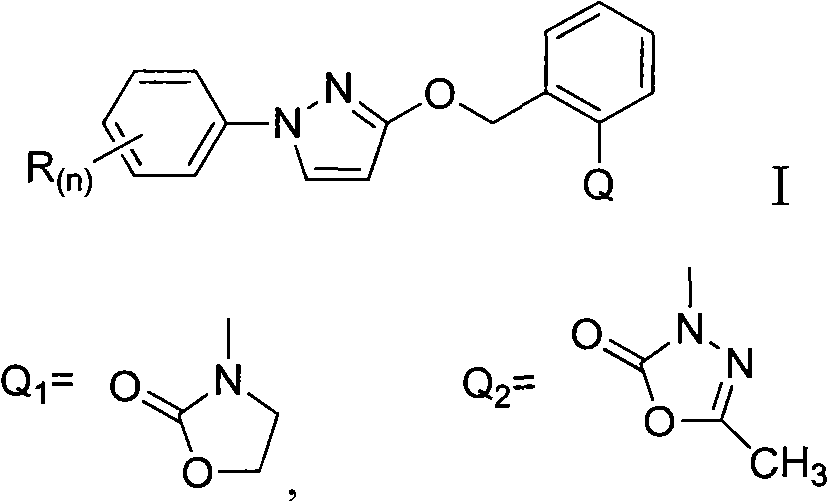
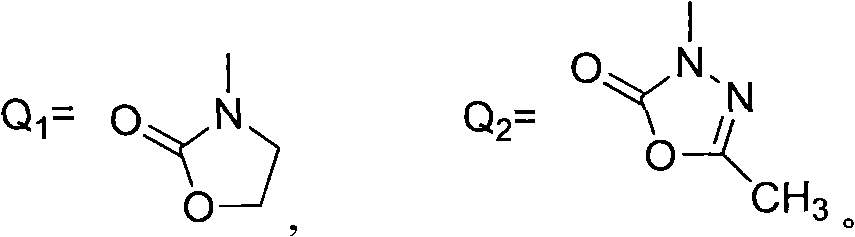Heterocyclic ketone-containing N-substituted phenyl pyrazole compound, preparation method thereof and application thereof in prevention and control of plant diseases and insect pests
A technology of phenylpyrazoles and compounds, applied in the field of N-substituted phenylpyrazoles and its preparation and control of plant diseases and insect pests, to achieve good insecticidal effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1: Synthesis of 3-(2-((1-phenyl-2H-pyrazole-3-oxyl)methyl)phenyl)oxazolinone-2-one (compound 1)
[0027]
[0028] Add 0.48g (3.0mmol) of compound (III-1), 0.62g (4.5mmol) of potassium carbonate, and 20mL of anhydrous acetone into a 100mL four-neck flask, stir for 0.5h at the reflux temperature of acetone (56°C), and then add 0.77g (3.00mmol) of compound (II-1) was dissolved in 15mL of acetone. After continuing to react at this temperature for 12h, it was left to cool and added 50mL of water to quench the reaction. Ethyl acetate (50mL×3) was extracted, the organic layer was washed with saturated sodium chloride solution, dried over anhydrous magnesium sulfate, and the solvent was evaporated under reduced pressure to obtain a yellow viscous liquid, which was separated and purified by column chromatography (silica gel filled, petroleum ether / ethyl acetate) to obtain a white solid with a yield of 88.5% and a melting point of 105-106°C.
[0029] NMR data ( 1 HN...
Embodiment 2
[0030] Example 2: 3-(2-((1-(4-chlorophenyl)-2H-pyrazole-3-oxyl group) methyl) phenyl) oxazolone-2-one (compound 2) synthesis
[0031]
[0032] Add 0.58g (3.0mmol) of compound (III-2), 0.62g (4.5mmol) of potassium carbonate, and 20mL of anhydrous acetonitrile into a 100mL four-neck flask, stir for 0.5h at the reflux temperature of acetonitrile (76°C), and then add 0.77g (3.00mmol) of compound (II-1) was dissolved in 15mL of acetonitrile. After continuing to react at this temperature for 18h, it was left to cool and added 50mL of water to quench the reaction. Ethyl acetate (50mL×3) was extracted, the organic layer was washed with saturated sodium chloride solution, dried over anhydrous magnesium sulfate, and the solvent was evaporated under reduced pressure to obtain a yellow viscous liquid, which was separated and purified by column chromatography (silica gel filled, petroleum ether / ethyl acetate) to obtain a white solid with a yield of 86.0% and a melting point of 138-139...
Embodiment 3
[0034] Example 3: 3-(2-((1-(3-chlorophenyl)-2H-pyrazole-3-oxyl)methyl)phenyl)oxazolinone-2-one (compound 3) synthesis
[0035]
[0036]0.58g (3.0mmol) of compound (III-3), 0.62g (4.5mmol) of potassium carbonate, and 20mL of anhydrous N,N-dimethylformamide were added to a 100mL four-necked flask, and dissolved in N,N-dimethylformamide Stir for 0.5 h at the reflux temperature (115° C.) of dimethylformamide, then add 0.77 g (3.00 mmol) of compound (II-1) in 15 mL of N,N-dimethylformamide solution, and continue the reaction at this temperature for 20 h. Let cool and add 50 mL of water to quench the reaction. Ethyl acetate (50mL×3) was extracted, the organic layer was washed with saturated sodium chloride solution, dried over anhydrous magnesium sulfate, and the solvent was evaporated under reduced pressure to obtain a yellow viscous liquid, which was separated and purified by column chromatography (silica gel filled, petroleum ether / ethyl acetate) to obtain a white solid wit...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com