Method for preparing (R)-styrene glycol by changing coenzyme specificity and stereoselectivity via site-directed mutagenesis
A technology of phenylethylene glycol and construction method, which is applied in the field of biocatalytic asymmetric transformation, and can solve the problems of insufficient substrate concentration, low optical purity, and expensive consumption, etc.
Active Publication Date: 2010-08-25
JIANGNAN UNIV
View PDF0 Cites 4 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Biological conversion to prepare optically pure (R)-phenylethylene glycol, usually using microbial whole cells or enzymes as catalysts, mainly including Bakers' yeast asymmetric reduction of 2-hydroxyacetophenone method, using epoxide hydrolase to catalyze Hydrolysis of racemic styrene oxide method, or the use of naphthalene dioxygenase (NDO) selective oxidation of styrene method, etc., the above methods have low optical purity of the product, the concentration of the substrate is not high enough, or the reaction process needs to consume expensive NADPH as a coenzyme to optimize reaction conditions and other shortcomings
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
Login to View More
Abstract
The invention discloses a (R)-styrene glycol preparation method that utilizes site-directed mutagenesis to alter coenzyme specificity and stereoselectivity, belongs to the technical field of biocatalytic asymmetric transformation, and provides a recombinant Escherichia coli strain BL21 / pETSCR6768 with the preservation CCTCC NO of M208079, and an asymmetric transforming preparation method of (R)-styrene glycol. The preparation method leads Ser in position 67 and His in position 68 of a (S)-specific carbonyl reductase to mutate to Asp, constructs recombinant plasmid pETSCR6768, and feeds the Escherichia coli, thus obtaining the recombinant strain E.coli BL21 / pETSCR6768 which leads the coenzyme specificity of (S)-specific carbonyl reductase to change from NADPH to NADH; furthermore, the stereoselectivity of the product is altered from (S)-styrene glycol to (R)-styrene glycol, and the preparation method provides an effective way for preparing (R)-styrene glycol in high efficiency and low cost and has significant meaning for recognizing the stereoselectivity of functional zymoprotein in the molecule and protein level.
Description
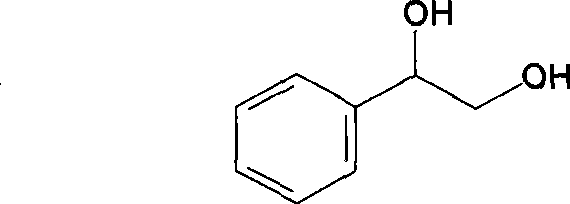
technical field A method for preparing (R)-phenylethylene glycol by changing coenzyme specificity and stereoselectivity by site-directed mutagenesis. The present invention relates to the use of protein engineering technology to transform amino acids at key sites in the enzyme protein structure, and to construct recombinant strains by means of genetic engineering. The method for efficiently preparing (R)-phenylethylene glycol and its application belong to the technical field of biocatalytic asymmetric conversion. Background technique The important role of chiral compounds in medicine, agriculture, industry and life has attracted more and more attention. Since the two enantiomers are different in pharmacology, toxicology and functional effects, the preparation of optically pure The chiral modular compounds are of great significance. The chemical structure of phenyl glycol is: Optically pure (R)-phenylethylene glycol is not only an indispensable and important chiral additiv...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Patents(China)
IPC IPC(8): C12N1/21C12P7/22C12N9/02C12N15/53C12N15/70C12R1/19
Inventor 张荣珍徐岩
Owner JIANGNAN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com