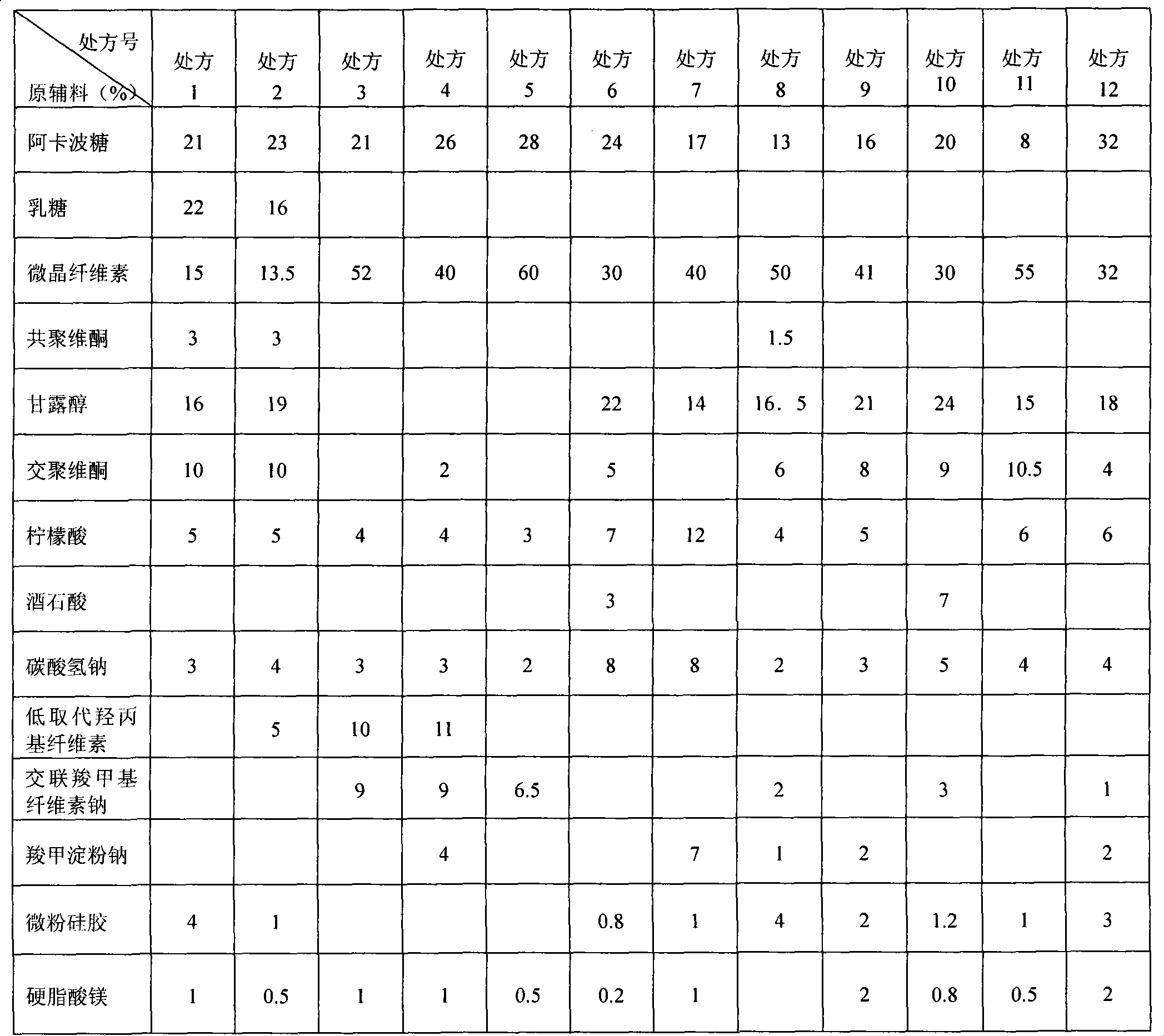
Pharmaceutical composition containing acarbose
A technology of acarbose and composition, which is applied in the field of pharmaceutical composition containing acarbose, and can solve the problems such as the inconvenience of taking ordinary tablets
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Acarbose: 50g
[0062] Microcrystalline Cellulose: 130g
[0063] Mannitol: 67g
[0064] Crospovidone: 33g
[0065] Citric acid: 17g
[0066] Sodium bicarbonate: 12g
[0067] Micropowder silica gel: 3.3g
[0068] Magnesium Stearate: 1.7g
[0069] Weighed according to the prescription, each material was passed through an 80-mesh sieve in advance, mixed well, and directly compressed into 1000 tablets.
Embodiment 2
[0071] Acarbose: 50g
[0072] Microcrystalline Cellulose: 180g
[0073] Mannitol: 67g
[0074] Copovidone: 5g
[0075] Crospovidone: 33g
[0076] Citric acid: 17g
[0077] Sodium bicarbonate: 12g
[0078] Micropowder silica gel: 3.3g
[0079] Magnesium Stearate: 1.7g
[0080] Weighed according to the prescription, each material was passed through an 80-mesh sieve in advance, mixed well, and directly compressed into 1000 tablets.
Embodiment 3
[0082] Acarbose: 50g
[0083] Microcrystalline cellulose: 90g
[0084] Mannitol: 67g
[0085] Crospovidone: 33g
[0086] Citric acid: 17g
[0087] Sodium bicarbonate: 12g
[0088] Micropowder silica gel: 3.3g
[0089] Magnesium Stearate: 1.7g
[0090] Weighed according to the prescription, each material was passed through an 80-mesh sieve in advance, mixed well, and directly compressed into 1000 tablets.
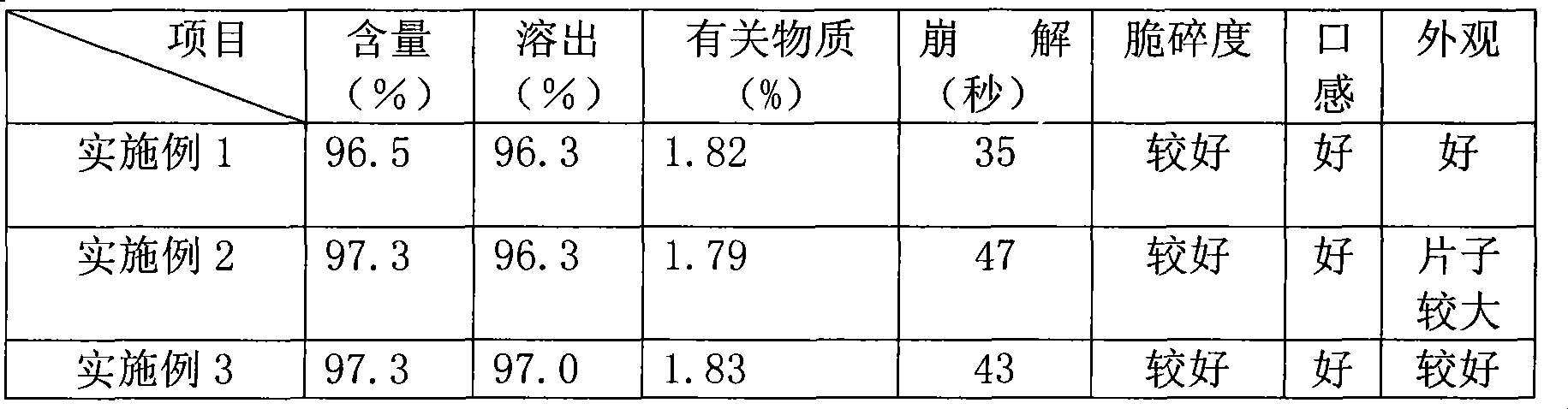
[0091] The embodiment is verified, and the results are shown in the following table:
[0092]
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com