TOLL like receptor 3 antagonists, methods and uses
An antagonist and receptor technology, applied in the field of Toll-like receptor 3 antagonists
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0226] Identification of anti-hTLR3 antagonist mAbs
[0227] Anti-hTLR3 antagonist mAbs that block signaling through the hTLR3 receptor are identified by cell-based screening assays. A library of hybridomas producing anti-hTLR3 mAb was generated in BALB / C mice using standard techniques (Kohler et al., 1976). Mice were immunized with hTLR3 by intradermal injection of plasmid DNA encoding amino acids 1-703 of hTLR3 (SEQ ID NO: 3). Amino acids 1-703 correspond to the predicted extracellular domain of hTLR3 (SEQ ID NO: 4). Initially, mice were injected with 10 μg of plasmid DNA, followed by a second 10 μg DNA injection 2 weeks later. Two weeks after the second 10 μg plasmid DNA injection, a booster injection of 15 μg DNA was administered to each mouse. Three days before B cell fusion, mice were injected intravenously with 15 μg of hTLR3 protein dissolved in phosphate buffered saline (PBS; 10 mM phosphate, 150 mM NaCl, pH 7.4). Spleens from immunized mice were then harvested ...
Embodiment 2
[0233] Effects of hTLR3 antagonists on IL-6, IL-8 and RANTES cytokines in human lung-derived cells Inhibition of child production
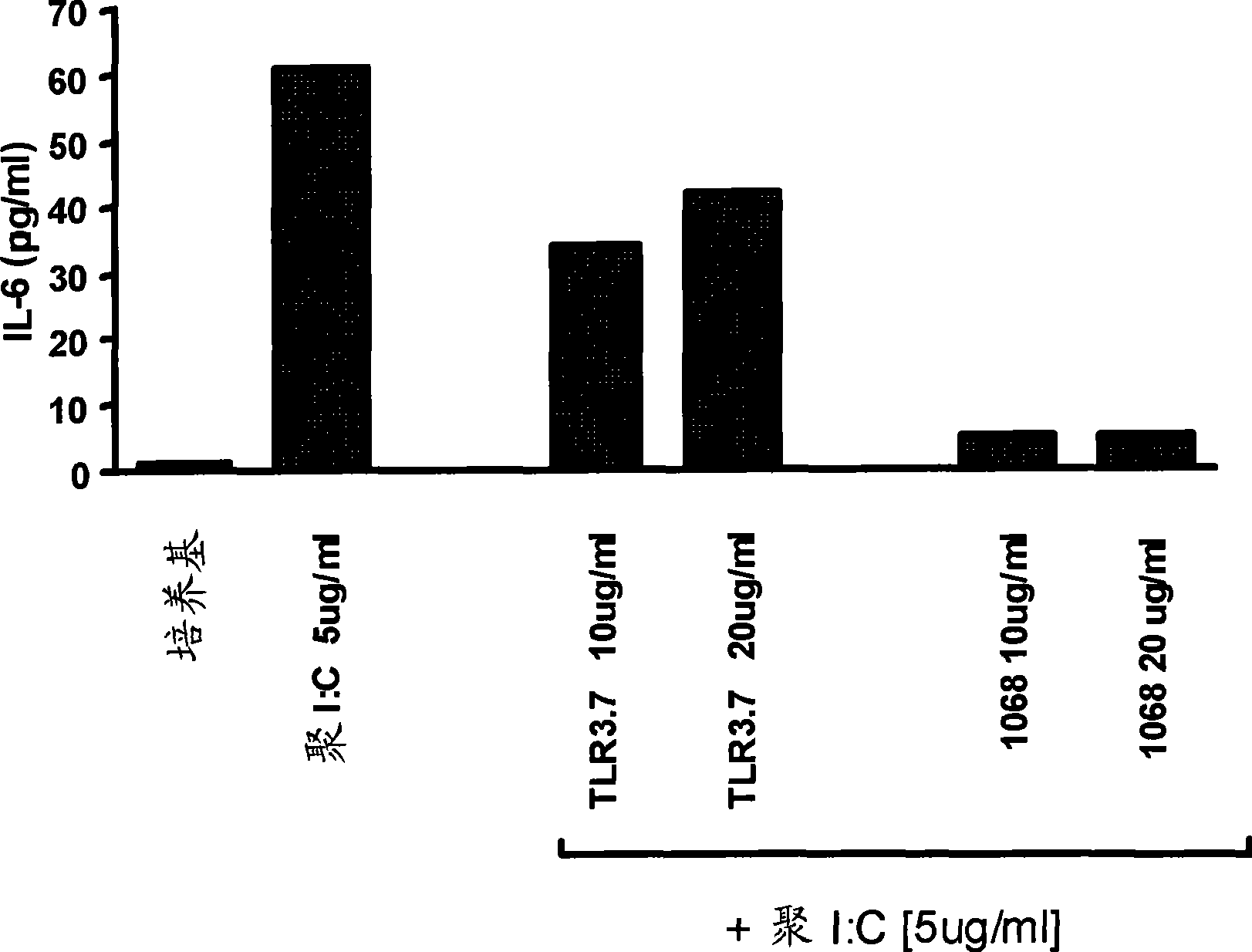
[0234] Such as image 3 , Figure 4 and Figure 5 As indicated, IL-6, TLR3 cells were detected by incubating A549-hTLR3 cells with 1068 mAb or TLR3.7 mAb at 37°C for 30 minutes, followed by the addition of 5 μg / ml poly(I:C) (Amersham Biosciences Corp., Piscataway, NJ). Cytokine assays specific for IL-8 and RANTES. After 24 hours, use Cytokine levels in cell culture supernatants were measured using the instrument (Luminex Corp., Austin, TX) and mAb-conjugated beads specific for IL-6, IL-8 or RANTES as appropriate. Follow the manufacturer's instructions for each cytokine Determination.
[0235] The results showed that the hTLR3 antagonist mAb 1068 inhibited hTLR3-mediated IL-6( image 3 ), IL-8 ( Figure 4 ) and RANTES ( Figure 5 ) cytokine production. However, hTLR3-specific murine mAb TLR3.7 (eBioscience, San Diego, CA) did not inhi...
Embodiment 3
[0237] Effect of hTLR3 antagonist on MIP1-α and IL-6 cells in primary human bronchial-epithelial cells Inhibition of factor production
[0238] hTLR3 antagonist mAb 1068 inhibits hTLR3-mediated MIP1-α( Figure 6 ) and IL-6 ( Figure 7 ) cytokine production. Such as Figure 6 or Figure 7 As indicated, primary human bronchial-epithelial cells were incubated with 1068mAb or non-specific polyclonal mouse IgG preparations at 37°C for 30 minutes, followed by the addition of 5 μg / ml poly(I:C) (Amersham Biosciences Corp., Piscataway, NJ). , MIP1-α and IL-6 specific cytokine assays were performed. After 24 hours, use Cytokine levels in cell culture supernatants were measured using the instrument (Luminex Corp., Austin, TX) and appropriate mAb-conjugated beads specific for MIP1-[alpha] or IL-6. Follow the manufacturer's instructions for each cytokine Determination. Primary human bronchio-epithelial cells were isolated from human tissue samples and cultured using standard ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com