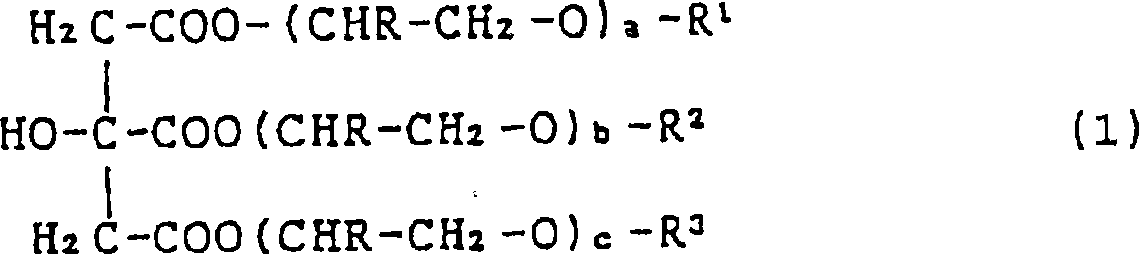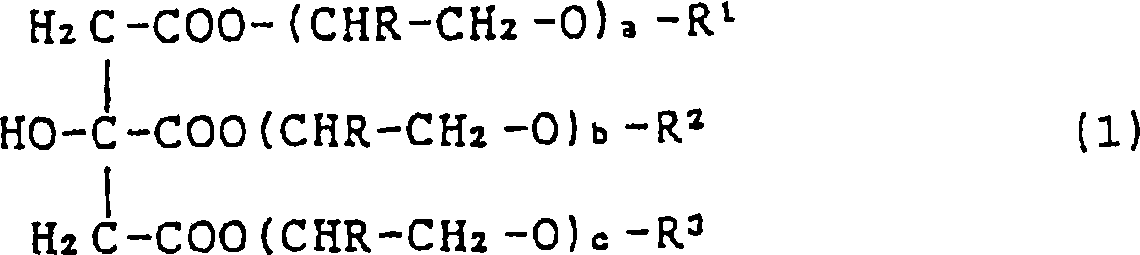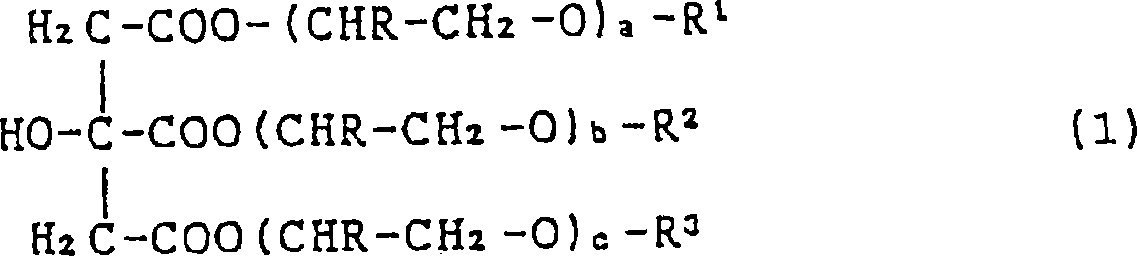Sulfo succinate of ester of citric acid, fatty alcohol and polyglycol ether, preparation and use thereof
A technology of sulfosuccinate and polyethylene glycol ether, applied in chemical instruments and methods, preparations for skin care, pharmaceutical formulations, etc., can solve the problems of not meeting the requirements of actual use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] 1.1 Esterification
[0059] In nitrogen, 377g (1.79mol) citric acid monohydrate, 723.6g (1.97mol) REWOPAL R LA 4 * and 1.3 g of hypophosphorous acid were introduced into a reaction vessel equipped with a stirrer, thermometer, water separator and condenser.
[0060] The mixture was heated to 140° C. under a nitrogen atmosphere with good stirring and maintained at this temperature for 2 hours. During this time, almost the theoretical amount of reaction water is distilled off. When the reaction mixture was cooled to 80°C, the pressure was slowly reduced to 20 mbar, at which pressure the reaction was completed at 140°C.
[0061] The acid value of the reaction product was 200.
[0062] * = product and trademark of REWO Chemische Werke Gmbh; C with 4 ethylene oxide (EO) units, OHN 153 12 / 14 Fatty alcohol ethoxylates.
[0063] 1.2 Ethoxylation
[0064] After adding 4 g of alkyldimethylamine, 784 g of the citric acid monoester obtained in Example 1.1 were reacted in...
Embodiment 2
[0078] 2.1 Esterification
[0079] In nitrogen, 210g (1mol) citric acid monohydrate, 507g (1.1mol) REWOPAL R LA6 * and 1.3 g of hypophosphorous acid were introduced into a reaction vessel equipped with a stirrer, thermometer, water separator and condenser.
[0080] The mixture was heated to 140° C. under a nitrogen atmosphere with good stirring and maintained at this temperature for 2 hours. During this time, almost the theoretical amount of reaction water is distilled off. The reaction mixture was cooled to 80 °C and a vacuum of 20 mbar was applied to complete the reaction. The acid number of the final product was 173.
[0081] * = product and trademark of REWO Chemische Werke Gmbh; C with 6 ethylene oxide (EO) units and an OHN of 130 12 / 14 Fatty alcohol ethoxylates.
[0082] 2.2 Ethoxylation
[0083] After adding 3 g of alkyldimethylamine, 610 g of the citric acid monoester obtained in Example 2.1 were reacted in batches at 90° C. with 116 g of ethylene oxide in a...
Embodiment 3
[0097] 3.1 Esterification
[0098] In nitrogen, 141g (0.67mol) citric acid monohydrate, 470g (0.73mol) REWOPAL R LA 10 * and 1 g of hypophosphorous acid were introduced into a reaction vessel equipped with a stirrer, a thermometer, a water separator and a condenser.
[0099] The mixture was heated to 140° C. under a nitrogen atmosphere with good stirring and maintained at this temperature for 2 hours. During this time, an almost theoretical amount of reaction water was distilled off (acid number of the product: 121). The reaction mixture was cooled to 80 °C and a vacuum of 20 mbar was applied to complete the reaction. The acid number of the final product was 113.
[0100]* = product and trademark of REWO Chemische Werke Gmbh; C with 10 ethylene oxide (EO) units and an OHN of 90 12 / 14 Fatty alcohol ethoxylates.
[0101] 3.2 Ethoxylation
[0102] After addition of 2.6 g of alkyldimethylamine, 513 g of the citric acid monoester obtained in Example 3.1 were reacted in ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Acid value | aaaaa | aaaaa |
| Hydroxyl value | aaaaa | aaaaa |
| Acid value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com