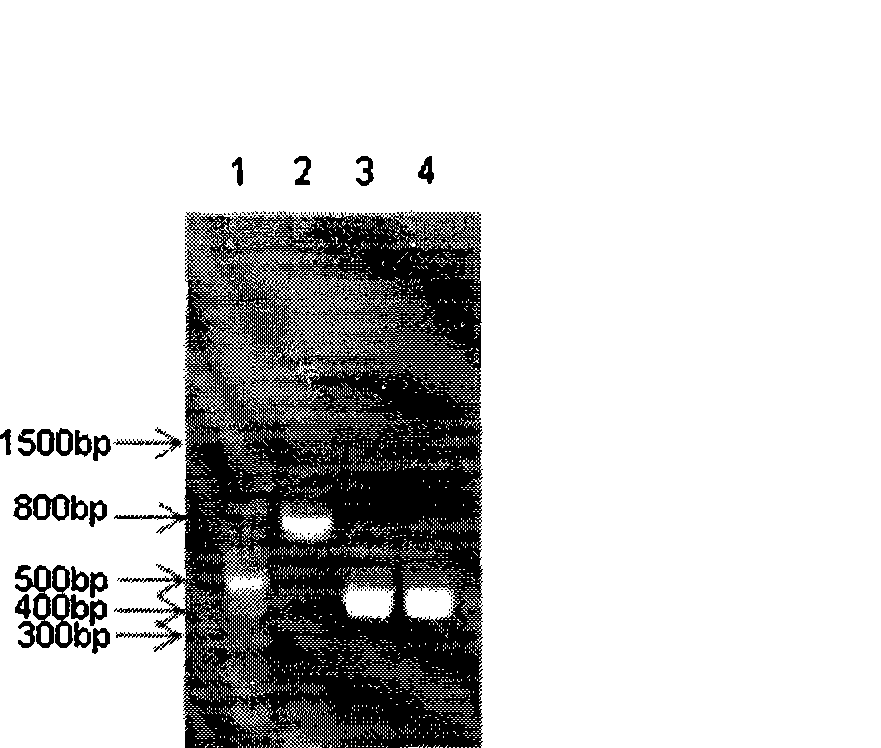
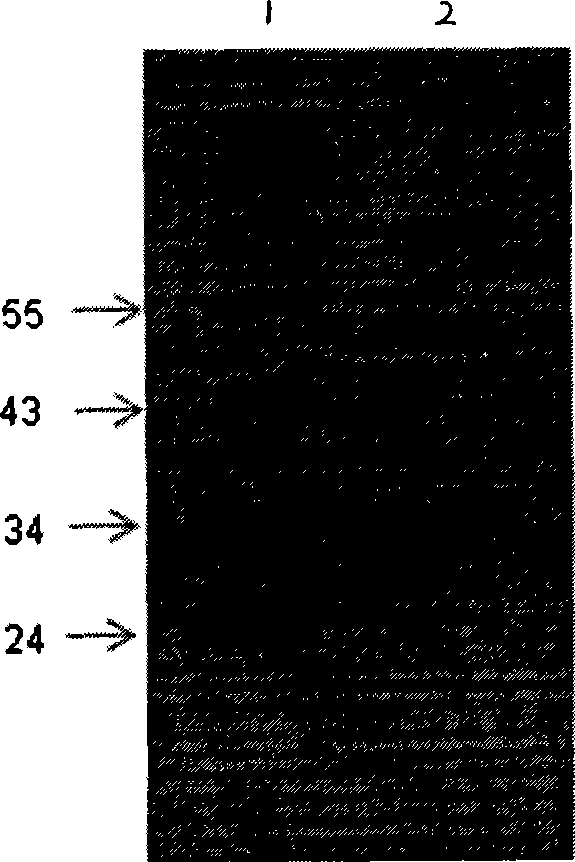
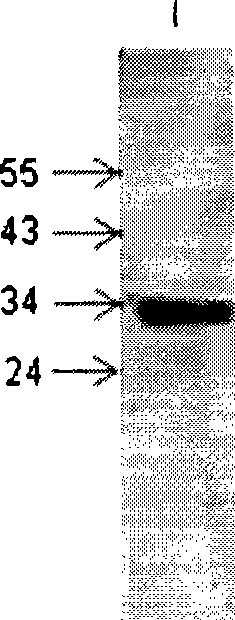
Heavy and light chain variable region gene of monoclonal antibody, encoding polypeptide thereof and use
A monoclonal antibody and gene-encoded technology, which can be used in antibody, gene therapy, genetic engineering, etc., can solve the problems of inability to resist EHEC0157 infection and low secretion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] 1. Preparation of monoclonal antibody S2C4 against EHEC Shiga toxin type II A subunit
[0015] (1) Immunization of Balb / c mice
[0016] Immunize 5-week-old female Balb / c mice with the fusion protein Stx2A-GST, 50ug / mouse, for the first immunization, mix 100ul antigen with an equal volume of Freund's complete adjuvant, and inject intraperitoneally; after the third week, the antigen and incomplete adjuvant Intraperitoneal immunization after mixing equal doses; the third immunization in the 5th week without adjuvant.
[0017] (2) Fusion of splenocytes and myeloma cells
[0018] One week before fusion, resuscitate mouse myeloma cells sp2 / 0 into OPTI-MEM medium (containing 10% fetal bovine serum) and place at 37°C, 5% CO 2 The cells were cultured in an incubator, and the cells were passaged once 3 days before fusion. On the day of fusion, harvest myeloma cells, count, and put 5×10 7 Myeloma cells were washed twice with serum-free medium for later use. 3-5 days after the...
Embodiment 2
[0165] LD 100 Determination: EHEC StxII toxin was diluted with sterilized PBS, intraperitoneally inoculated with 6-week-old BALB / c mice, and within 12 days of the experiment, the lowest dose of toxin that could kill all mice in the test group was 5 ng / mouse.
[0166] The mice were first intraperitoneally inoculated with 5ng / mouse of toxin, and 16 hours later, the intraperitoneal inoculation of S2C4 single-chain antibody ScFv, the dosage was 60, 30, 15, 8, 4ug / mouse, respectively, with S2C4 full molecule antibody and PBS as Positive, negative reference. The number of dead mice was recorded every day, and the whole experiment ended on the 12th day. It was found that when the dose of S2C4 single-chain antibody ScFv was 60-15ug / mouse, the mice could be completely protected from toxin attack; while the dose of ScFv was 8 and 4ug / mouse, the protective efficiency was only 60%. % and 20%, the results are shown in Table 1 and Table 2.
[0167] animal grouping number of a...
Embodiment 3
[0173] Get the crude extracts of three subtypes of EHEC StxII toxin: StxIIc, StxIId and StxIIvha were respectively intraperitoneally inoculated with 6-week-old BALB / c mice (100ul / only), 16 hours later, intraperitoneally inoculated with S2C4 10ug / mouse, recorded every day The number of dead mice, the whole experiment ended on the 12th day. It was found that, like StxII, S2C4 could completely neutralize the toxic effects of the three subtypes of StxII and protect mice from toxin challenge. The results are shown in Table 3.
[0174]
[0175] table 3
[0176] Conclusion: S2C4 has a strong neutralizing spectrum, and the epitope it targets may be quite conserved between StxII and its subtypes, which indicates that S2C4 is a very promising antibody drug candidate for the treatment of EHEC infection.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Relative molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com