Method for preparing mucosal graft
A technology of grafts and mucosa, applied in the field of medicine and biomedical engineering, can solve the problem of limited source of stem cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
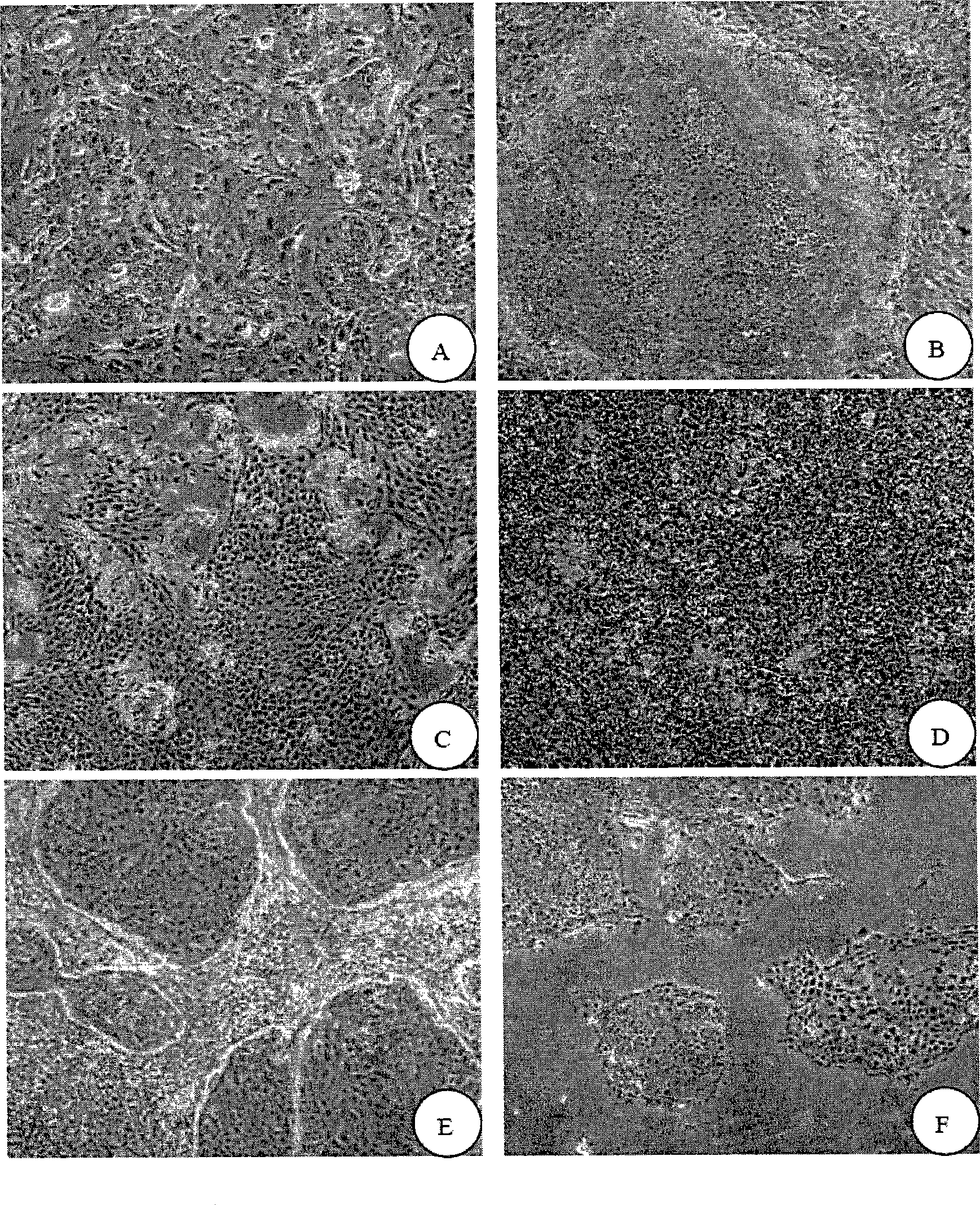
[0096] Isolation and in vitro culture of oral mucosal keratinocytes (OKC)
[0097] 1. Preparation of 3T3 feeder layer cells
[0098] NIH3T3 cells (purchased from the Institute of Cell Biology, Chinese Academy of Sciences) were used as trophoblast cells.
[0099]Take the frozen 3T3 cells and quickly rewarm them at 37°C. Mix and wash in DMEM, centrifuge at 1500 rpm for 5 minutes, take the cell pellet and repeat once. After counting, they were inoculated in a 10 cm diameter petri dish with DMEM culture solution containing 10% fetal bovine serum. When the 3T3 cells reached 70% confluency, they were washed with PBS three times, and treated with 10 ug / ml mitomycin C for 4 hours. Wash with PBS to stop the treatment, add 0.25% trypsin-0.02% EDTA to digest into a single cell suspension.
[0100] After centrifugation, count at 0.5×10 6 The density of / ml was inoculated in a 10cm-diameter petri dish, added with FAD conditioned medium, and cultivated overnight in a 37°C, 5% CO2 incub...
Embodiment 2
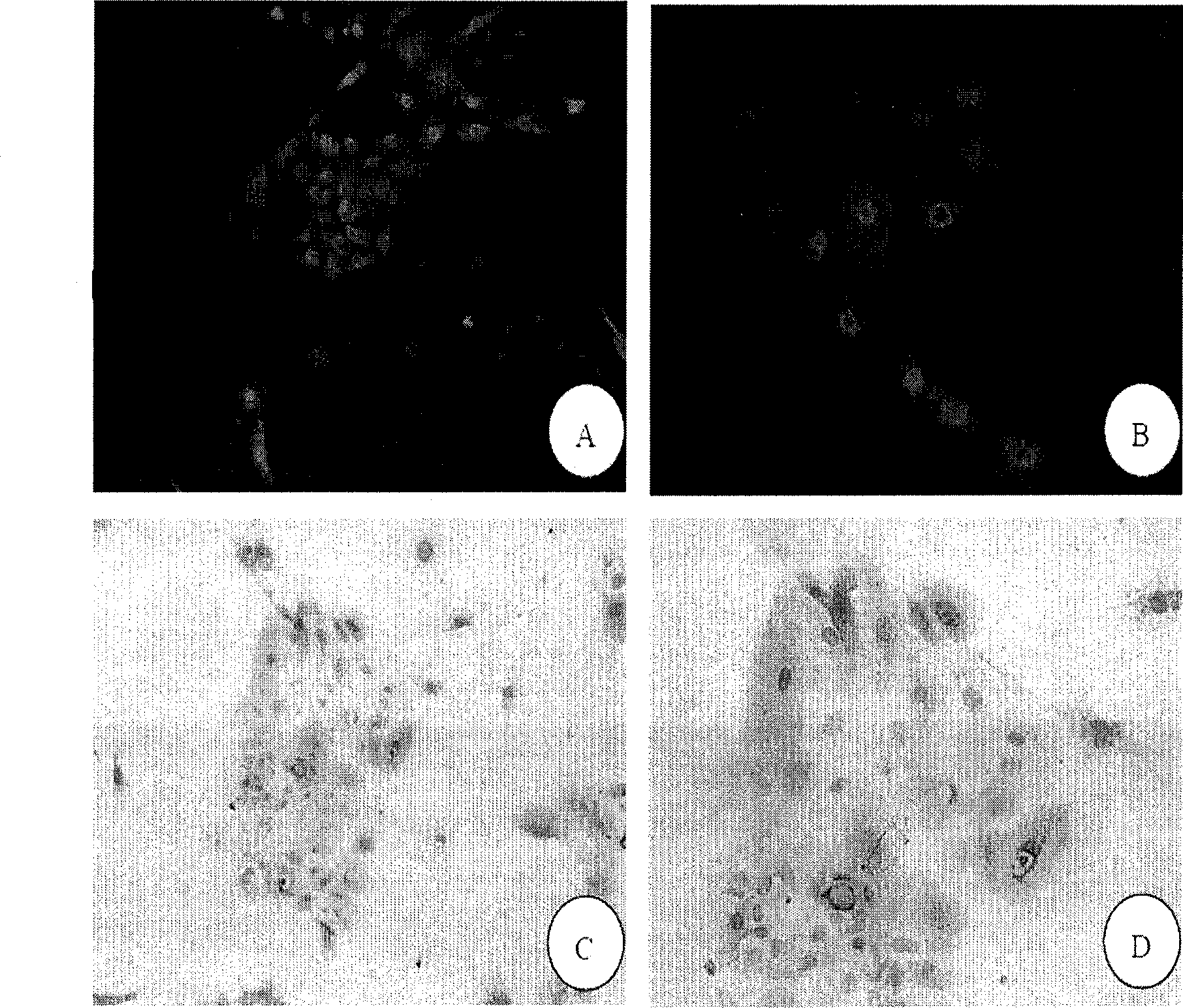
[0120] Identification of oral mucosal keratinocytes
[0121] 1.0 KC cell immunofluorescence detection
[0122] The p2 oral mucosal epithelial cells cultured in Example 1 were inoculated in a culture dish with a sterilized cover glass, and the cell crawl was taken out from the culture dish for detection by cell immunofluorescence. The specific process is as follows:
[0123] Cell slides were rinsed with PBS, 2 minutes x 5 times;
[0124] Fix with 4% paraformaldehyde for 10-15 minutes, rinse with PBS for 2 minutes x 5 times;
[0125] Add dropwise 0.25% TritonX-100, 5% DMSO-PBS at room temperature for 10 minutes;
[0126] Rinse with PBS for 5 minutes x 3 times;
[0127] Add 10% goat serum dropwise and place in a humid box at 37°C for 30 minutes;
[0128] After absorbing the goat serum, dry it;
[0129] Add mouse anti-human monoclonal antibody cytokeratin 14 (cytokeratin14, Abcam) dropwise, the working concentration is 1:100, and put it in a humid box at 4°C overnight;
[0...
Embodiment 3
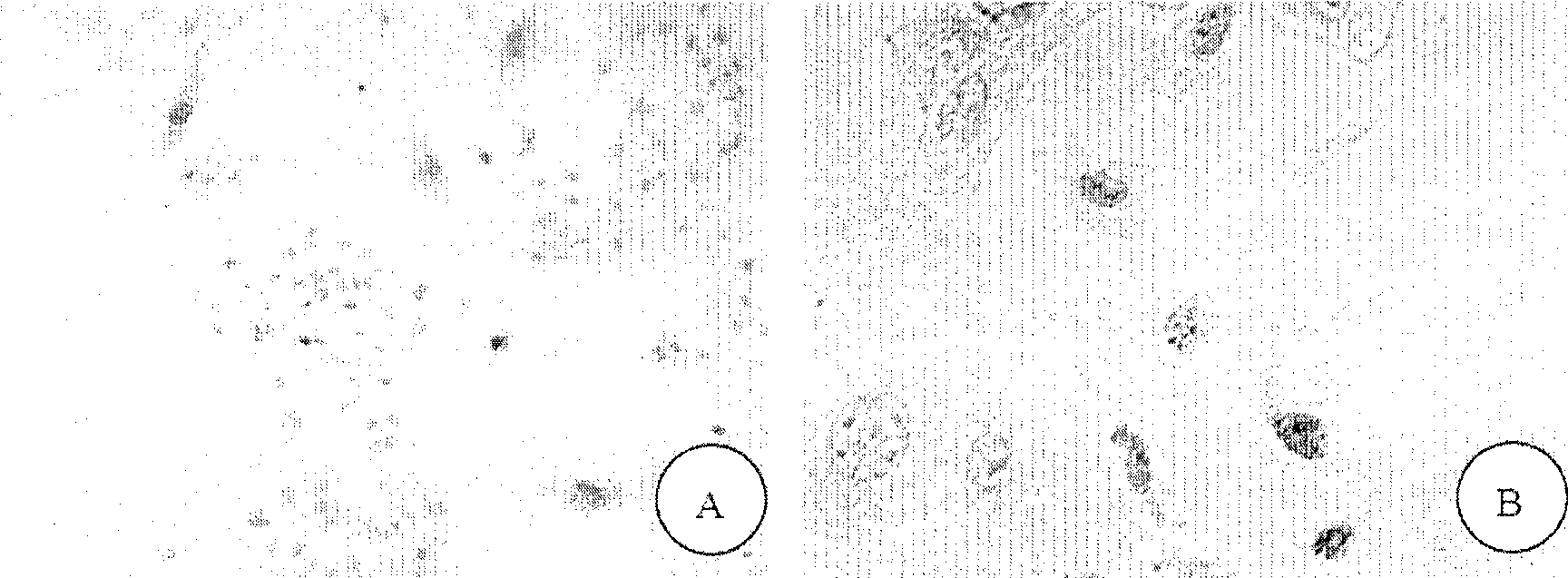
[0146] BrdU labeling and identification of oral mucosal keratinocytes
[0147] 1. BrdU labeling of oral mucosal keratinocytes
[0148] Take nearly 50% confluent P2 generation OKC cells obtained in Example 1, add 1:1000 5-bromo 2-deoxyuridine (5-bromo 2-deoxyuridine, BrdU, Sigma) solution to the culture medium, at 37 ° C, Place in a 5% CO2 incubator for 48 hours until the OKC cells reach 80-90% cell confluence.
[0149] Discard the culture medium containing BrdU, wash with PBS repeatedly, add 0.25 trypsin-0.02% EDTA to digest into a single cell suspension.
[0150] Inoculate on clean and sterilized glass slides for identification.
[0151] 2. Immunohistochemical identification of cells (BrdU)
[0152] The cell slides were fixed with 4% paraformaldehyde for 10 minutes, and washed 3 times with PBS.
[0153] Add 0.25% TritonX-100, 5% DMSO-PBS at room temperature for 10 minutes; rinse with PBS for 5 minutes x 3 times; add 0.75 / 1.5%-PBS H 2 o 2 37°C for 15 minutes; rinse with ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com