Methods and compositions for treating neurodegenerative disorders and alzheimer's disease and improving normal memory
An active and presenilin technology, applied in biological testing, animal/human proteins, instruments, etc., can solve the problems of disrupting the stability of β-catenin, defective intracellular transport, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
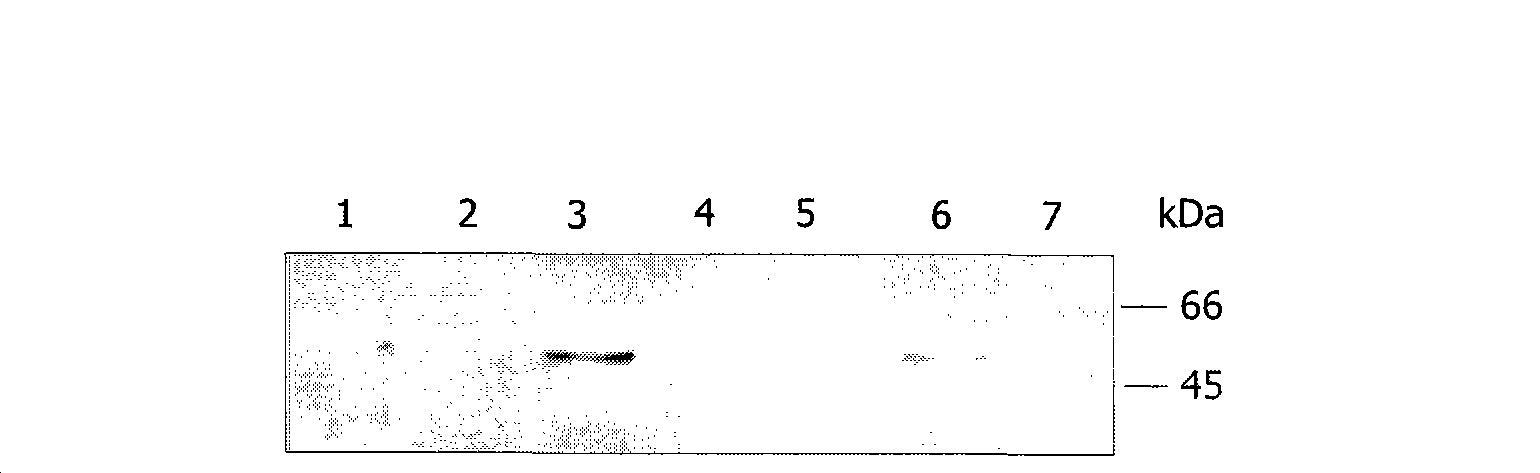
[0121] G-protein Gα in pcDNA3 was purchased from UMR cDNA Resource Center, Rolla, MO oA and Gα oB cDNAs. Full-length human PS-1 and PS-2 cDNAs were cloned by PCR in pcDNA3 as described. Tailless constructs of PS-1 and PS-2 were constructed in pcDNA3 in which only the cytoplasmic domain of PS-1 or PS-2 following the last TM-domain was deleted (the construct contained PS- amino acids 1-430 of 1 and amino acids 1-410 of PS-2).
[0122] Cell culture: ES (PS-1 - / - / PS-2 - / - ) cells were cultured according to published protocols.
[0123] Transfection: ES (PS-1 - / - / PS-2 - / - ) was transiently transfected with 15 μg of full-length human PS-1 or PS-2 pcDNA and the desired G-protein cDNA using the lipofectamine (Invitrogen) method. Briefly, the lipofectamine-DNA solution was left at room temperature for 30 min, mixed with sufficient serum-free medium and added to the cells, cytoplasmic at 37°C in CO 2 After 5 hours of incubation in an incubator, the medium was supplemented wit...
Embodiment 2
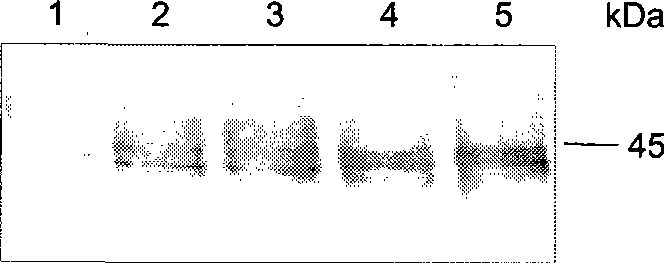
[0142] ES PS double nude cells were cultured and plated overnight. Cells were transfected with the pcDNA3 construct of full-length human β-APP cDNA using lipofectamine (Invitrogen) according to the manufacturer's protocol. DAMI cells were cultured and transfected with pcDNA3 or pcDNA3 constructs of full-length human PS-1 or PS-2 cDNA.
[0143] Affinity purified polyclonal rabbit anti-Ptyr antibody (Maher et al., 1985) was used for Western blot analysis, a gift from Dr. Elena Pasquale. A mouse monoclonal anti-Ptyr antibody (4G10; Upstate Biotechnology, Lake Placid, NY) was used in the ELISA analysis. Mouse monoclonal antibody to human pp60c-src (anti-Src, clone GD11) and rabbit polyclonal antibody to Lyn (anti-Lyn) were purchased from Upstate Biotechnology. Rabbit polyclonal antibody to Fyn (anti-Fyn, sc-16) was purchased from Santa Cruz Biotechnology, Santa Cruz, CA. The first rat anti-human PS-1 monoclonal antibody MAb #1563 directed against the N-terminal domain of PS-2 w...
Embodiment 3
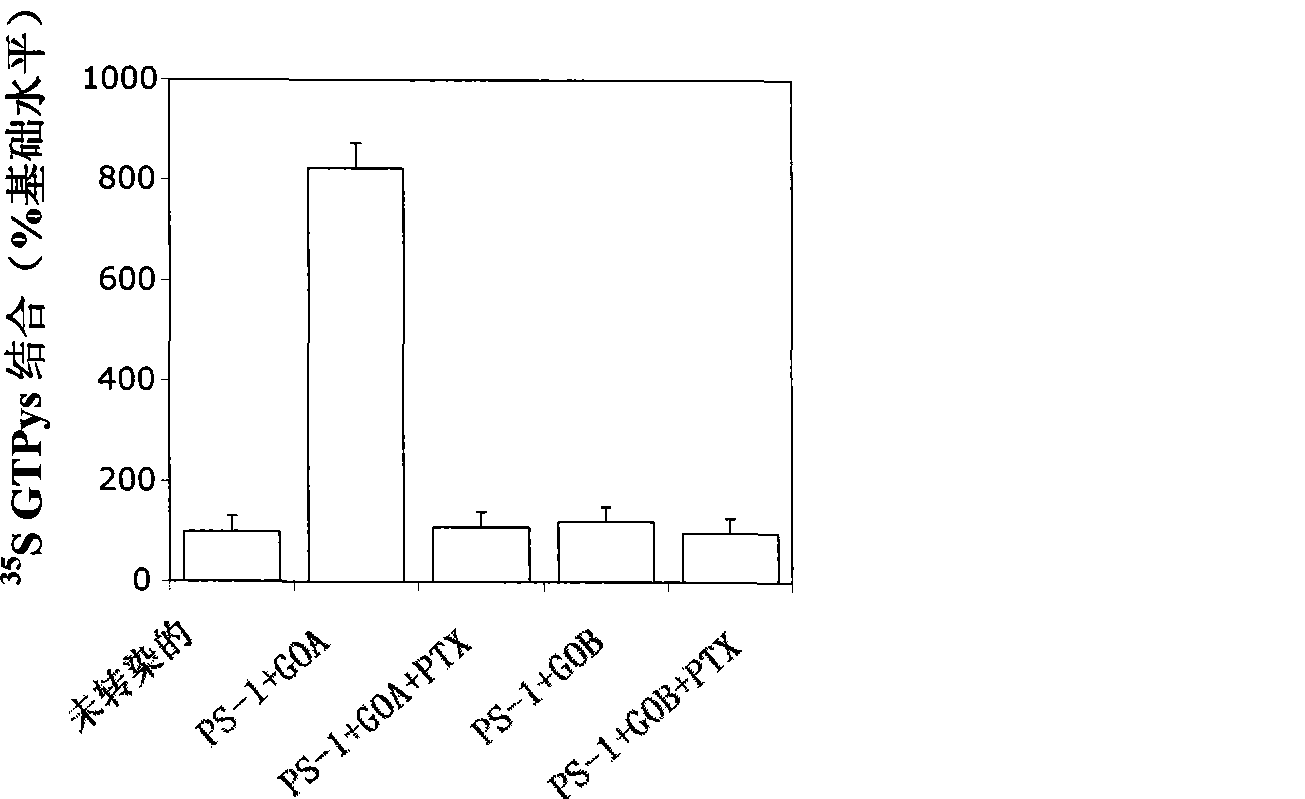
[0171] The following data demonstrate that G-proteins bind to endogenous PS-1 and PS-2 in extracts of mouse frontal cortex. GTPγS dissolution / extraction buffer [50mM HEPES / NaOH pH 7.4, 1mM EDTA, 1mM DTT, 1% Triton-X100, 60mM octyl glucoside, 1× protease inhibitor cocktail (1uM phenylmethanesulfonyl fluoride, 1ug / mL anti Protease, 0.1 ug / mL pepstatin A, 0.1 ug / mL leupeptin)] to make 20% homogenate of wild mouse forehead. Measured on untreated, PTx-treated, and PS-1 and PS-2 immunodepleted extracts[ 35 S] GTPγS binding.
[0172] For untreated samples, make 100 μL of 100 μg extract in GTPγS lysis / extraction buffer and mix with an equal volume of GTPγS buffer B (50 mM HEPES / NaOH pH 7.4, 40 μM GDP, 50 mM MgCl 2 , 100 mM NaCl) to a total volume of 200 μL. Reaction with 50nM[ 35 S] GTPyS (1250 Ci / mMol; Perkin Elmer) was started and incubated at room temperature for 60 minutes. By adding 10X stop buffer (100mM Tris-HCl, pH 8.0, 25mM MgCl 2 , 100mM NaCl, 20mM GTP) to stop the rea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com