Method of forming polyalkene substituted carboxylic acid compositions
A polyalkene, carboxylic acid technology, applied in the field of polyalkene-substituted carboxylic acid, anhydride or ester composition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
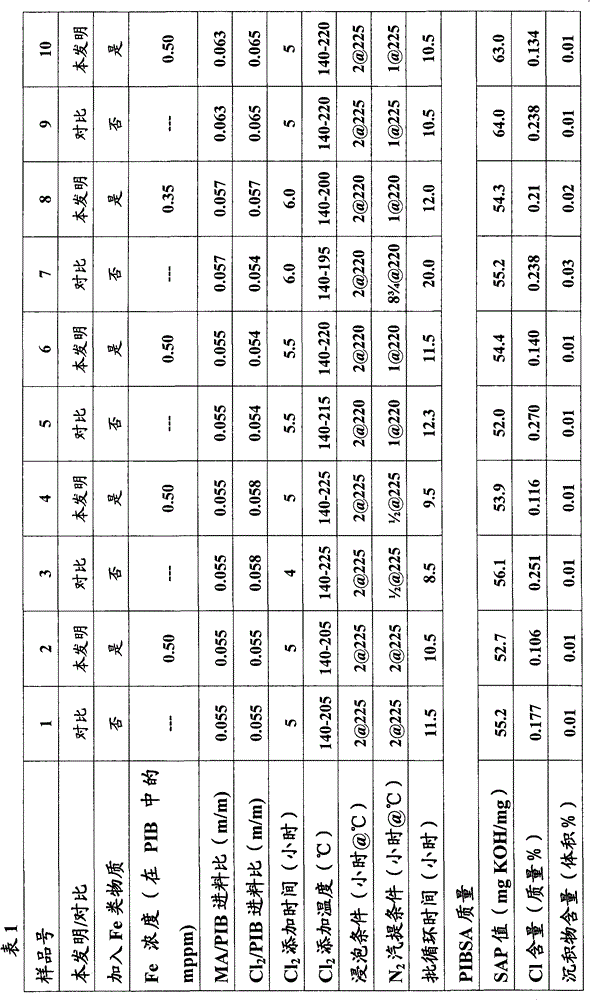
[0050] In order to confirm the effect of the method of the present invention, by making 2225M n Polyisobutene (PIB) and maleic anhydride (MA) were reacted in a simultaneous chlorination / maleization reaction under the following conditions in the presence and absence of specified amounts of polyisobutene-soluble iron salts (in Isopar-L solvent Iron(III) neodecanoate; Fe concentration in solution was 6% by mass) to form a series of polyisobutylene succinic anhydride (PIBSA) products with various functionality / SAP values. The SAP value, chlorine content and sediment content of the resulting PIBSA products were then measured and compared. The results are shown in Table 1 as follows:
[0051]
Embodiment 2
[0054] In order to confirm the adverse effect of higher metal content on the formation of PIBSA product, by making 2225 M n Polyisobutene (PIB) and maleic anhydride (MA) in a simultaneous chlorination / maleization reaction under the following conditions (i) in the absence of polyisobutene-soluble iron salts (iron(III) neodecanoate) and (ii ) in the presence of an iron salt soluble in polyisobutylene to provide more than 2 ppm iron (mass ppm in PIB) reacted to form a PIBSA product with the indicated functionality / SAP values. The SAP value, chlorine content and sediment content of the active ingredient (AI) of the resulting PIBSA product were then measured and compared. The results are shown in Table 2 below:
[0055] Table 2
[0056] sample number 11 12 Invention / comparison Compared Compared Add Fe species no yes Fe concentration (mppm in PIB) --- 3.0 MA / PIB feed ratio (m / m) 0.065 0.065 Cl 2 / PIB feed ratio (m / m) ...
Embodiment 3
[0060] In order to use other metal salts soluble in polyisobutylene to confirm the effect of the method of the present invention, by making 2225M n Polyisobutene (PIB) and maleic anhydride (MA) were soluble in the indicated feed ratios (feed ratios of reactants) in a simultaneous chlorination / maleation reaction under the following conditions in the presence and absence of In the case of nickel and copper salts of polyisobutylene (Ni(II)-2-ethylhexanoate and Cu(II)-2-ethylhexanoate), the polyisobutylene succinic anhydride (PIBSA) product is formed. The SAP value, chlorine content and sediment content of the resulting PIBSA products were then measured and compared. The results are shown in Table 3 below:
[0061] table 3
[0062] sample number 13 14 15 16 17 Invention / comparison Compared this invention this invention this invention this invention Add metal substances no yes yes yes yes metal substance --- Ni N...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com