Compositions and methods for modulating vascular development
An angiogenesis and anti-vascular technology, applied in the field of compositions regulating vascular development
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0354] v. Preparation of antibody-drug conjugates
[0355] In an antibody-drug conjugate (ADC), an antibody (Ab) is coupled to one or more drug moieties (D) via a linker (L), for example, about 1 to about 20 drug moieties per antibody . The ADC of general formula I can be prepared through several routes using organic chemical reactions, conditions and reagents known to those skilled in the art, including: (1) the nucleophilic group of the antibody reacts with a divalent linker reagent through a covalent bond to form Ab-L, which subsequently reacts with the drug moiety D; and (2) the nucleophilic group of the drug moiety reacts via a covalent bond with a divalent linker reagent to form D-L, which subsequently reacts with the nucleophilic group of the antibody. Additional methods for preparing ADCs are described herein.
[0356] Ab-(L-D)p I
[0357] A joint may consist of one or more joint components. Exemplary linker building blocks include 6-maleimidocaproyl ("MC"), male...
Embodiment 1
[0396] Example 1: Materials and methods
[0397] The following materials and methods were used in the examples.
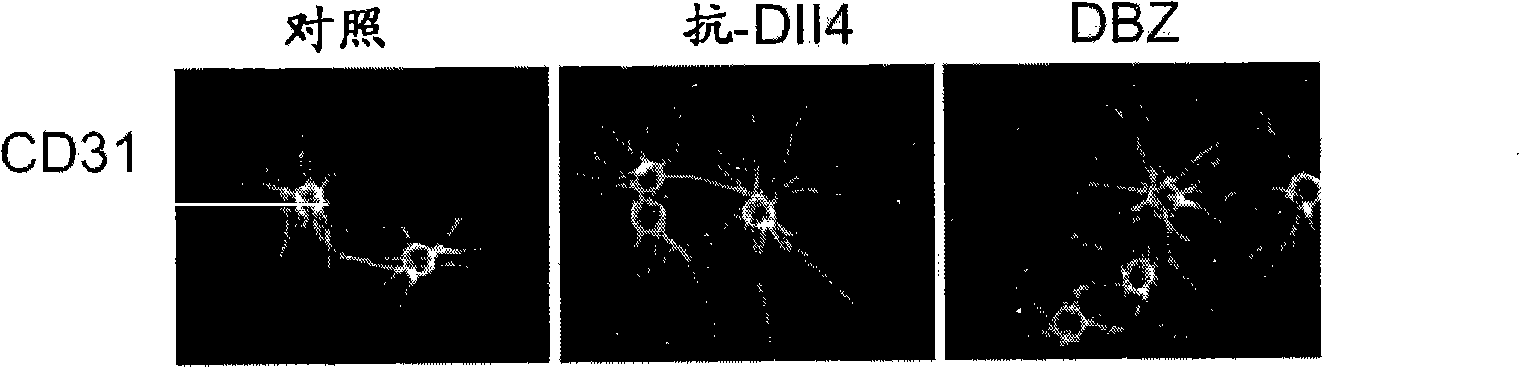
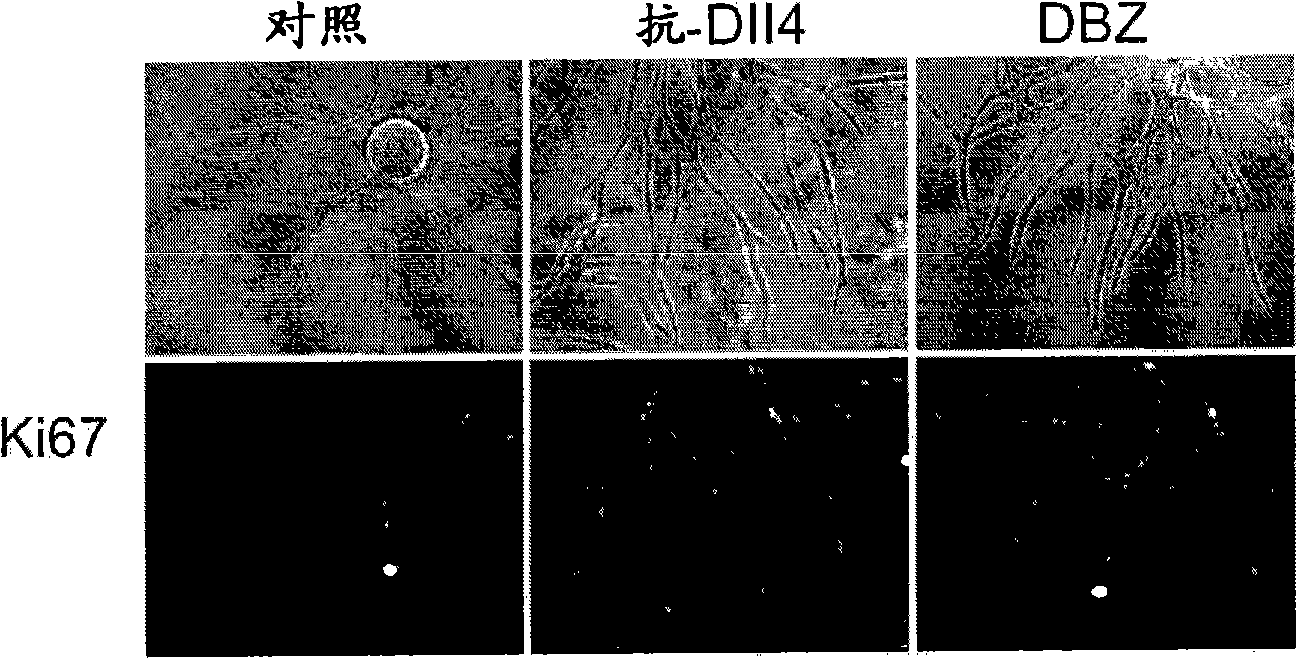
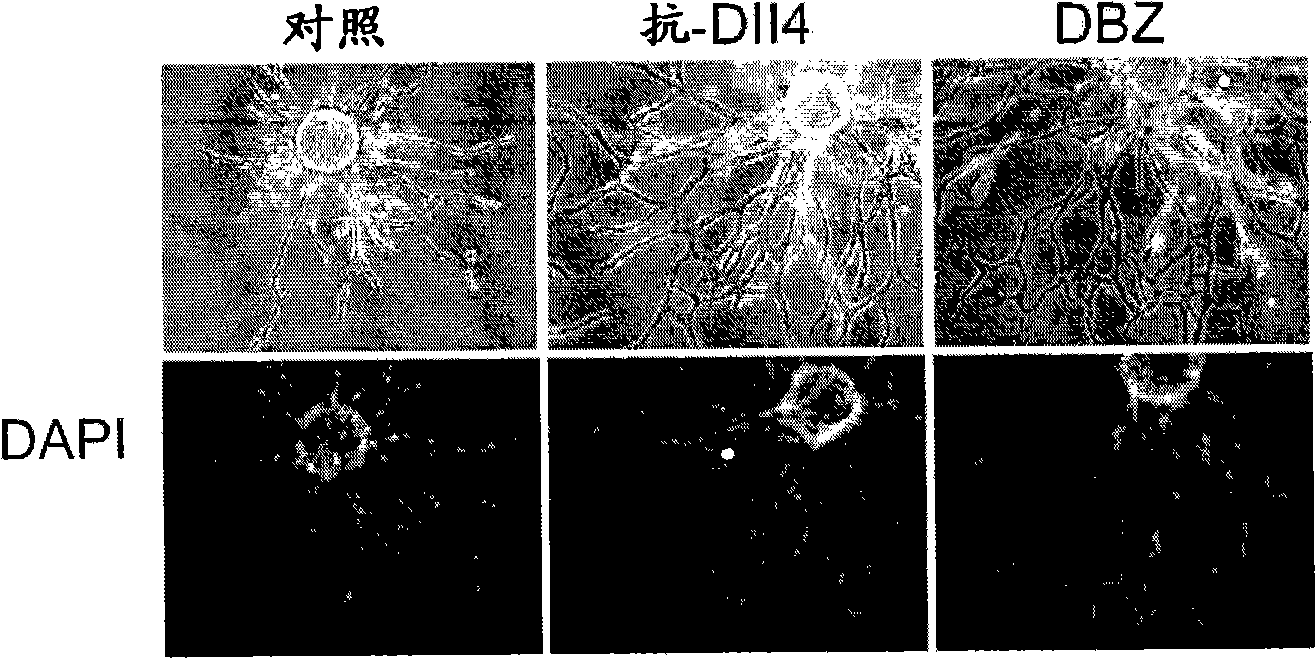
[0398] HUVEC fibrin gel bead assay. Details of the HUVEC fibrin gel bead assay have been described (Nakatsu, M.N. et al., Microvasc Res 66, 102-12 (2003)). Briefly, Cytodex™3 beads (Amersham Pharmacia Biotech) were coated at 350-400 HUVEC / bead. Embed approximately 200 HUVEC-coated beads into the fibrin clot in one well of a 12-well tissue culture plate. will be 8 x 10 4 SF cells were plated on top of the clot. Assays were terminated between days 7 and 9 for immunostaining and imaging. In some experiments, HUVEC shoots were visualized by staining with biotin-anti-CD31 (clone WM59, eBioscience) and streptavidin-Cy3. For HUVEC nuclei staining, fibrin gels were fixed overnight in 2% paraformaldehyde (PFA) and stained with 4',6-diamidino-2-phenylindole (DAPI, Sigma). For Ki67 staining, fibrin gels were treated with 10X trypsin-EDTA for 5 min to remove upper SF, n...
Embodiment 2
[0426] Example 2: Generation of phage anti-DLL4 antibodies
[0427] A synthetic phage antibody library was constructed on a single framework (humanized anti-ErbB2 antibody, 4D5) by introducing diversity within the complementarity-determining regions (CDRs) of the heavy and light chains (Lee, C.V. et al., J Mol Biol 340, 1073- 93 (2004); Liang, W.C. et al., J Biol Chem 281, 951-61 (2006)). For the His-tagged human DLL4 (amino acids 1-404) immobilized on the Maxisorp immunoplate, the unimmunized ( ) plate panning of the library. After four rounds of enrichment, clones were randomly picked and specific binders were identified using phage ELISA. The resulting hDLL4-binding clones were further screened with His-tagged murine DLL4 protein to identify cross-species clones. For each positive phage clone, the heavy and light chain variable regions were subcloned into a pRK expression vector engineered to express the full-length IgG chain. The heavy and light chain constructs were ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com