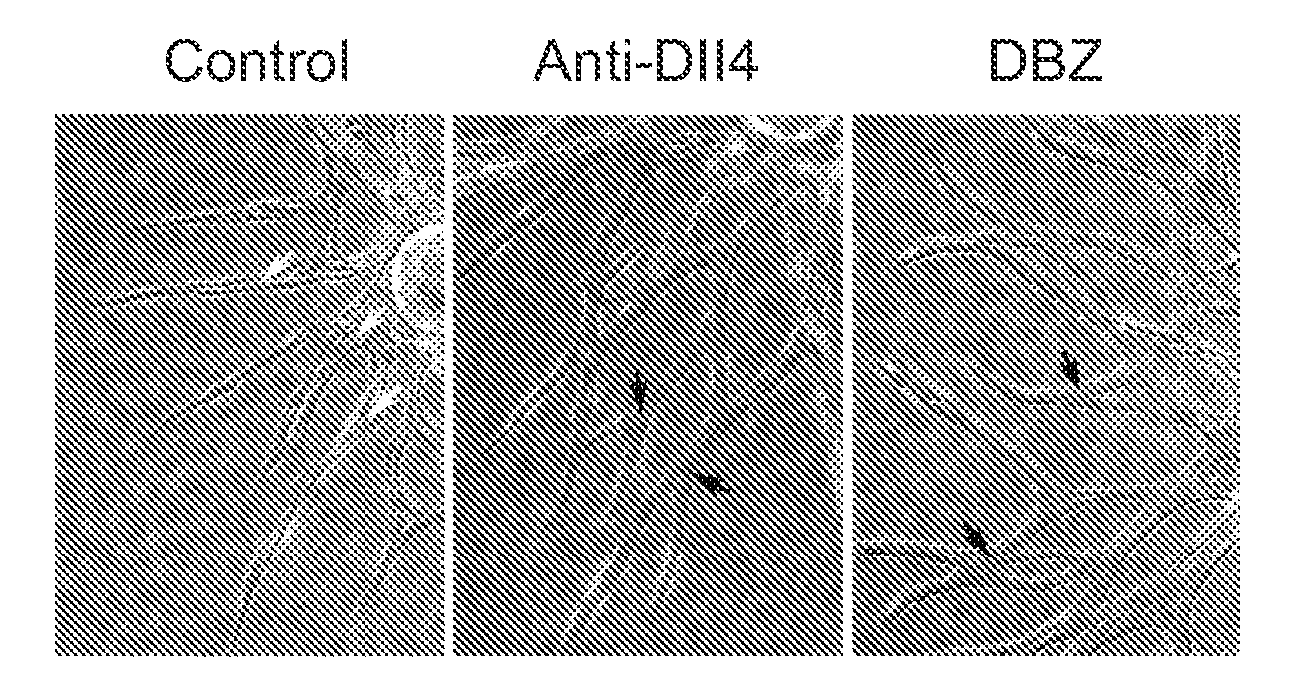
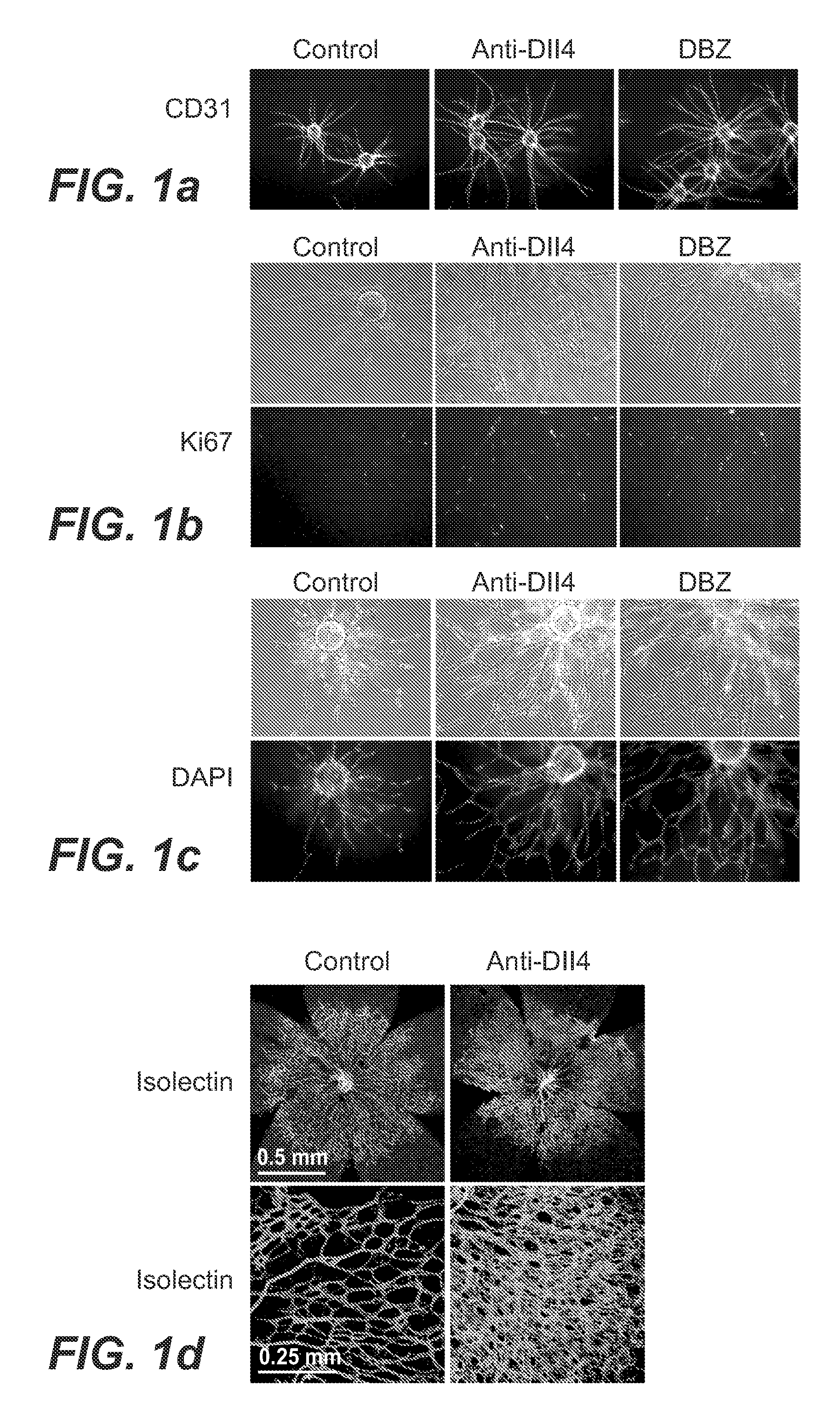
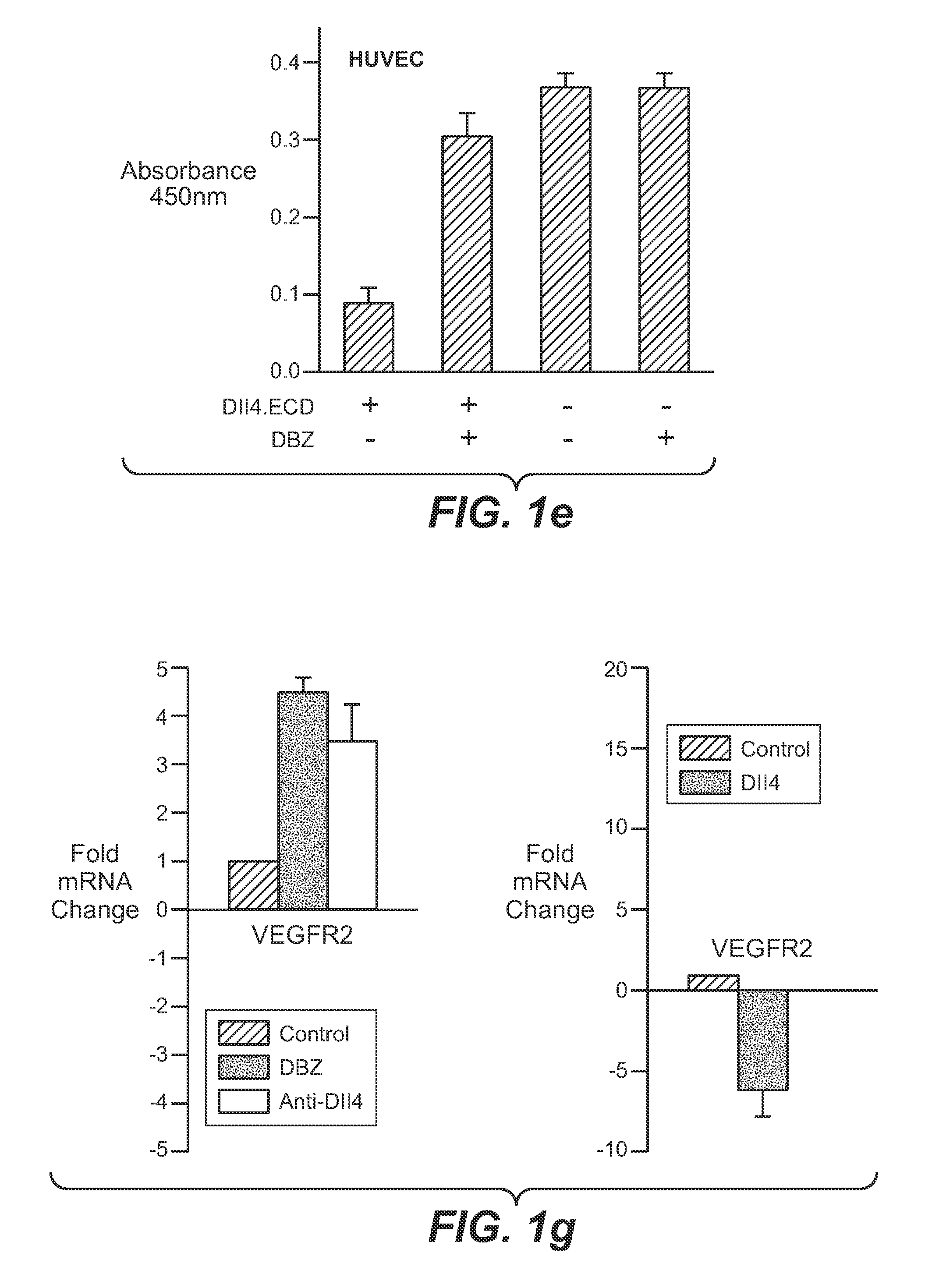
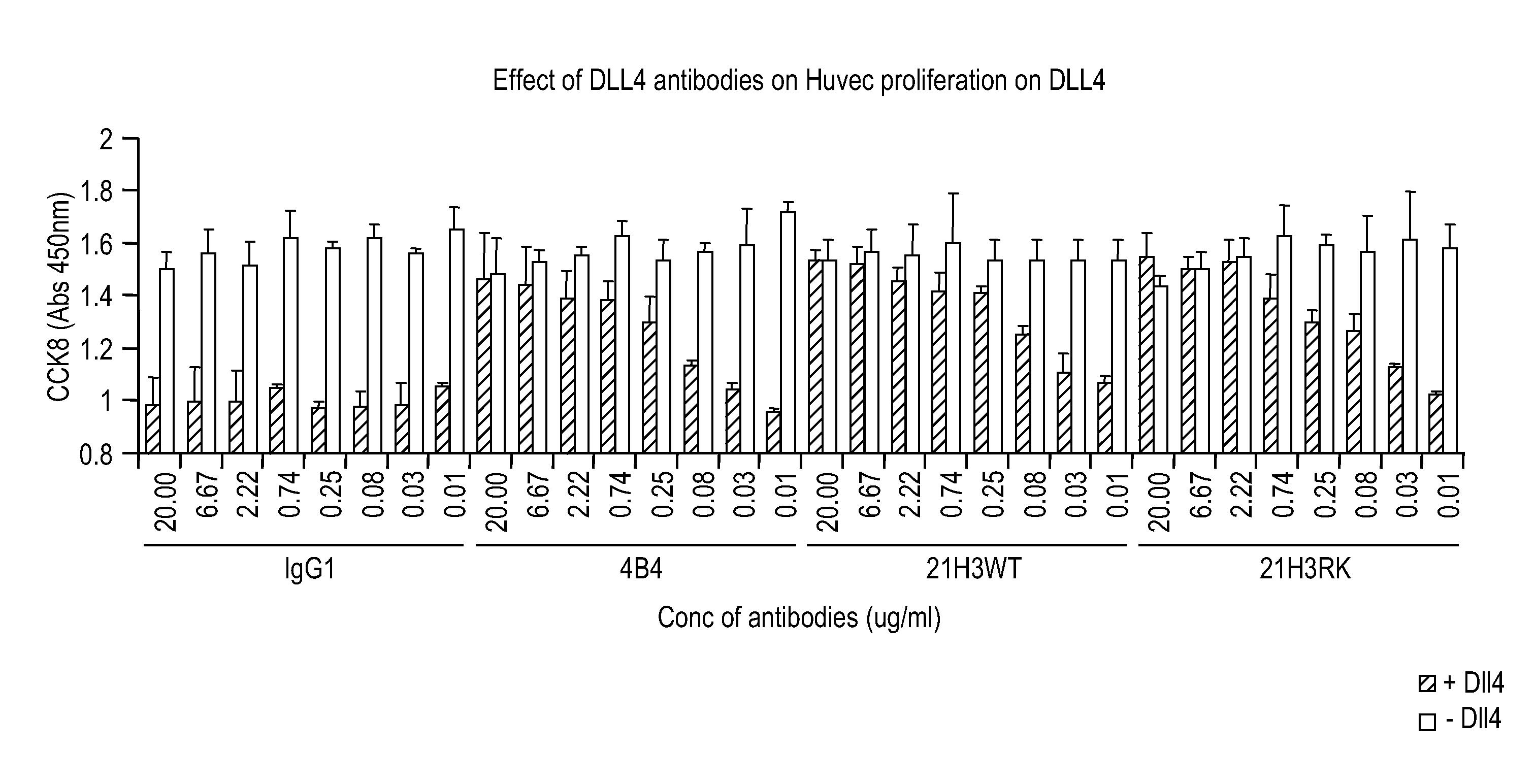
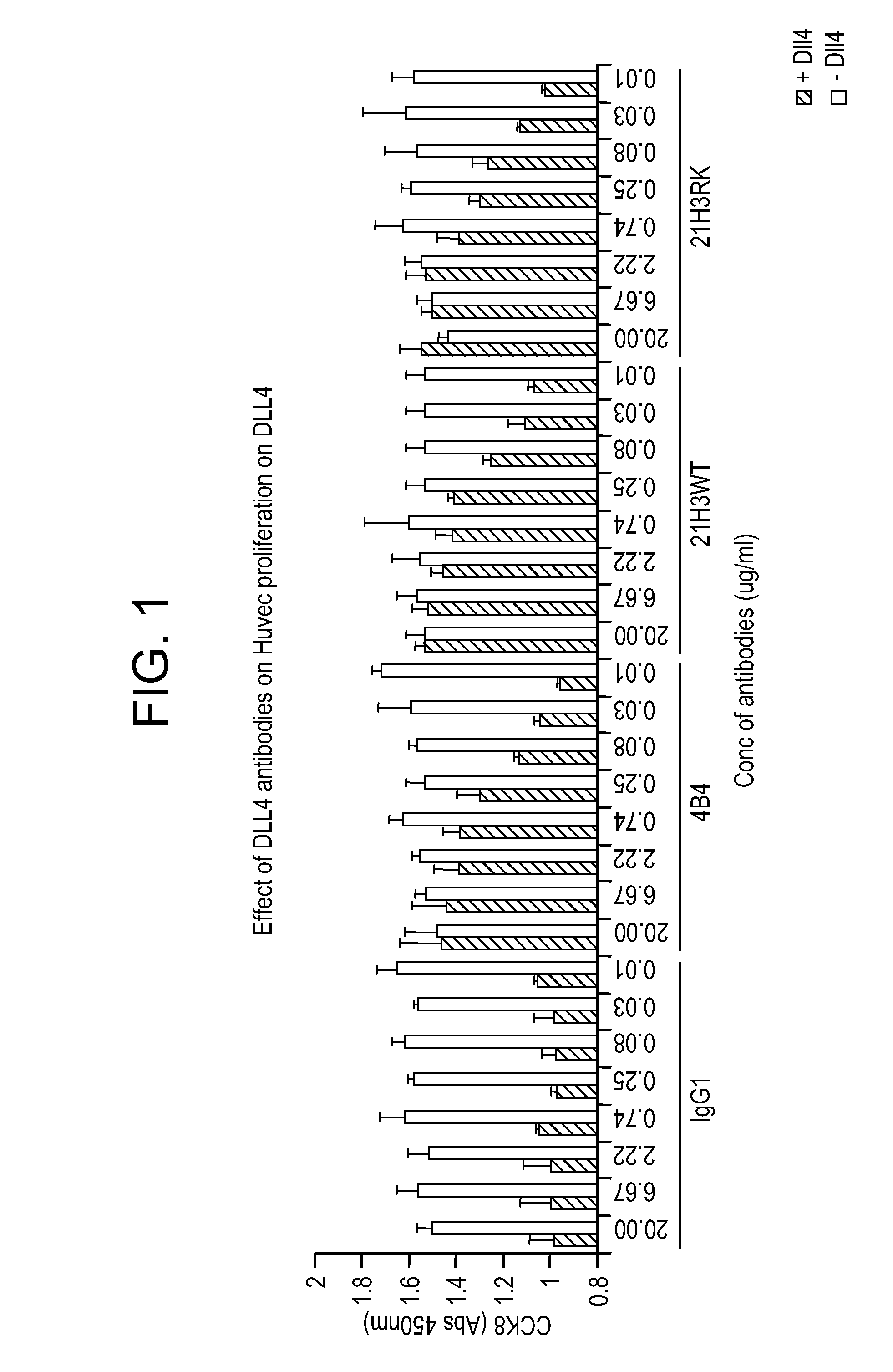
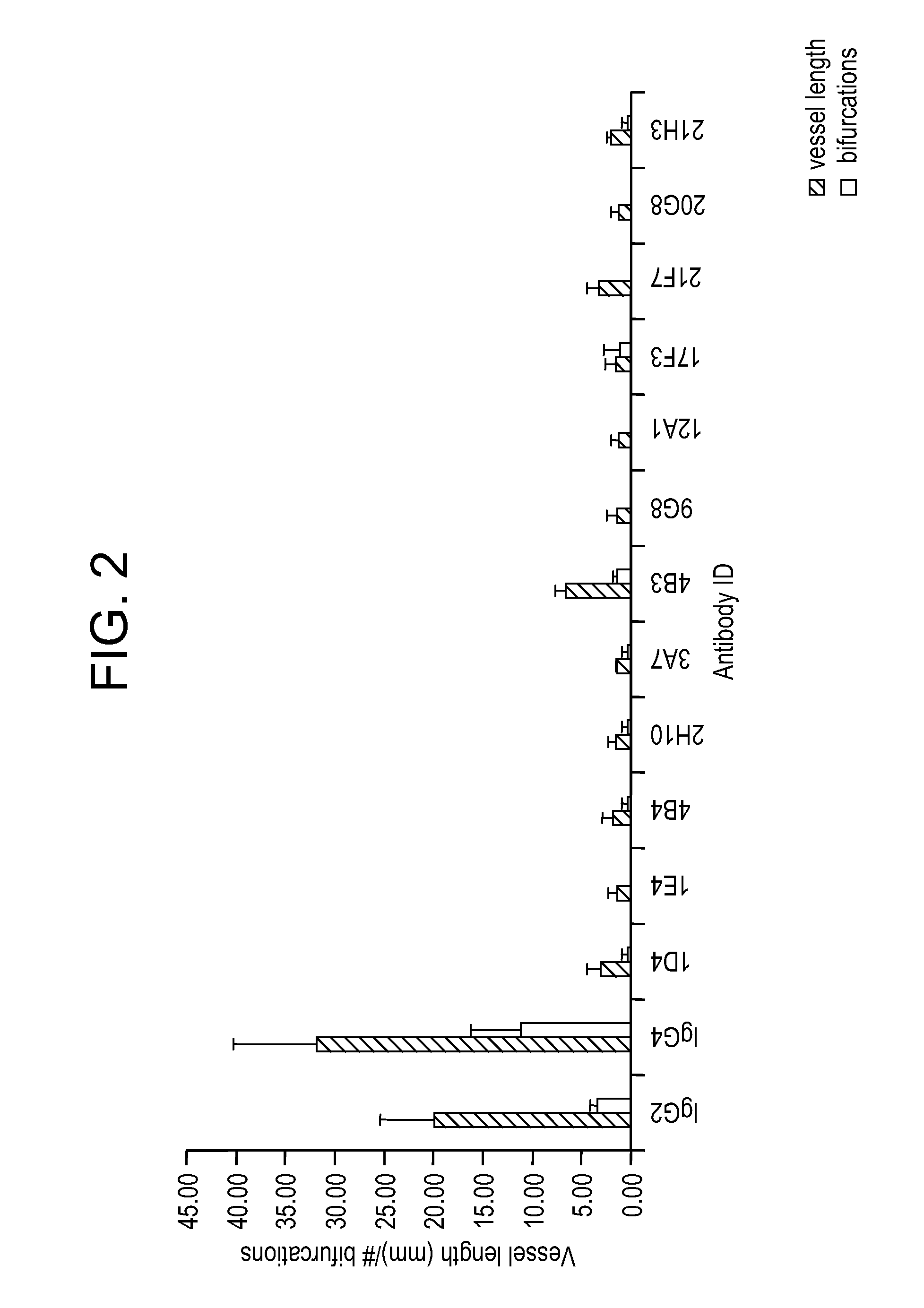
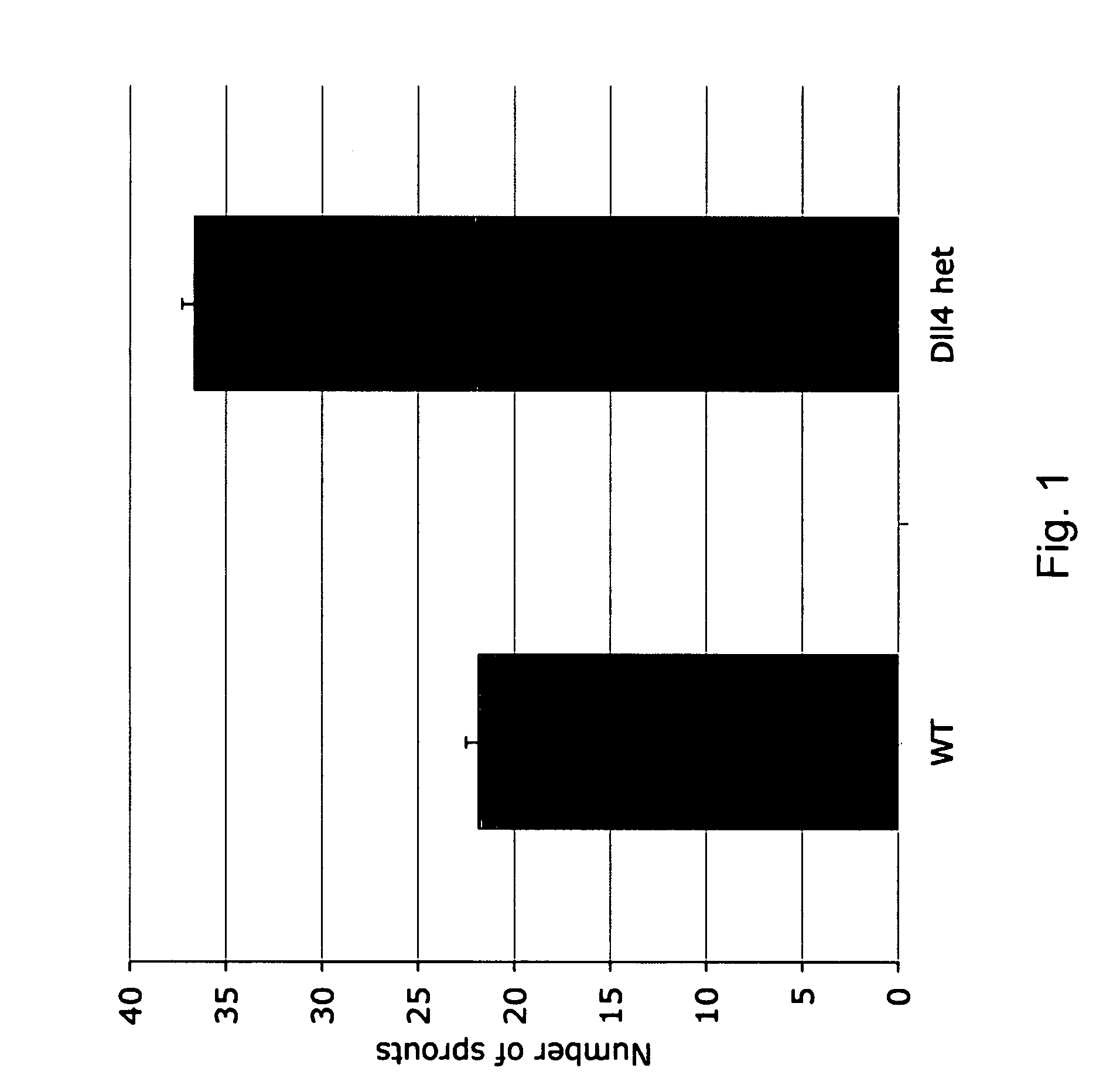
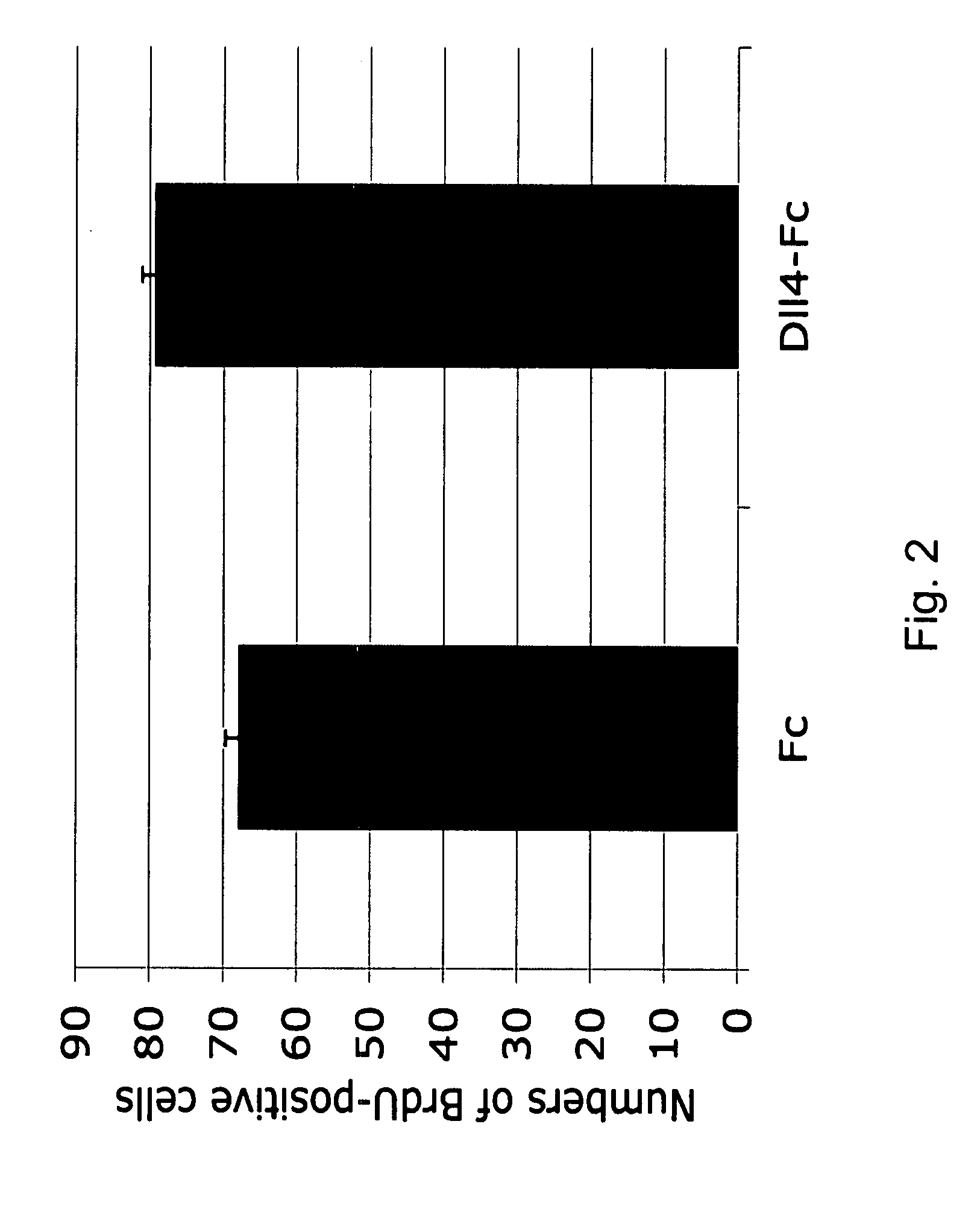
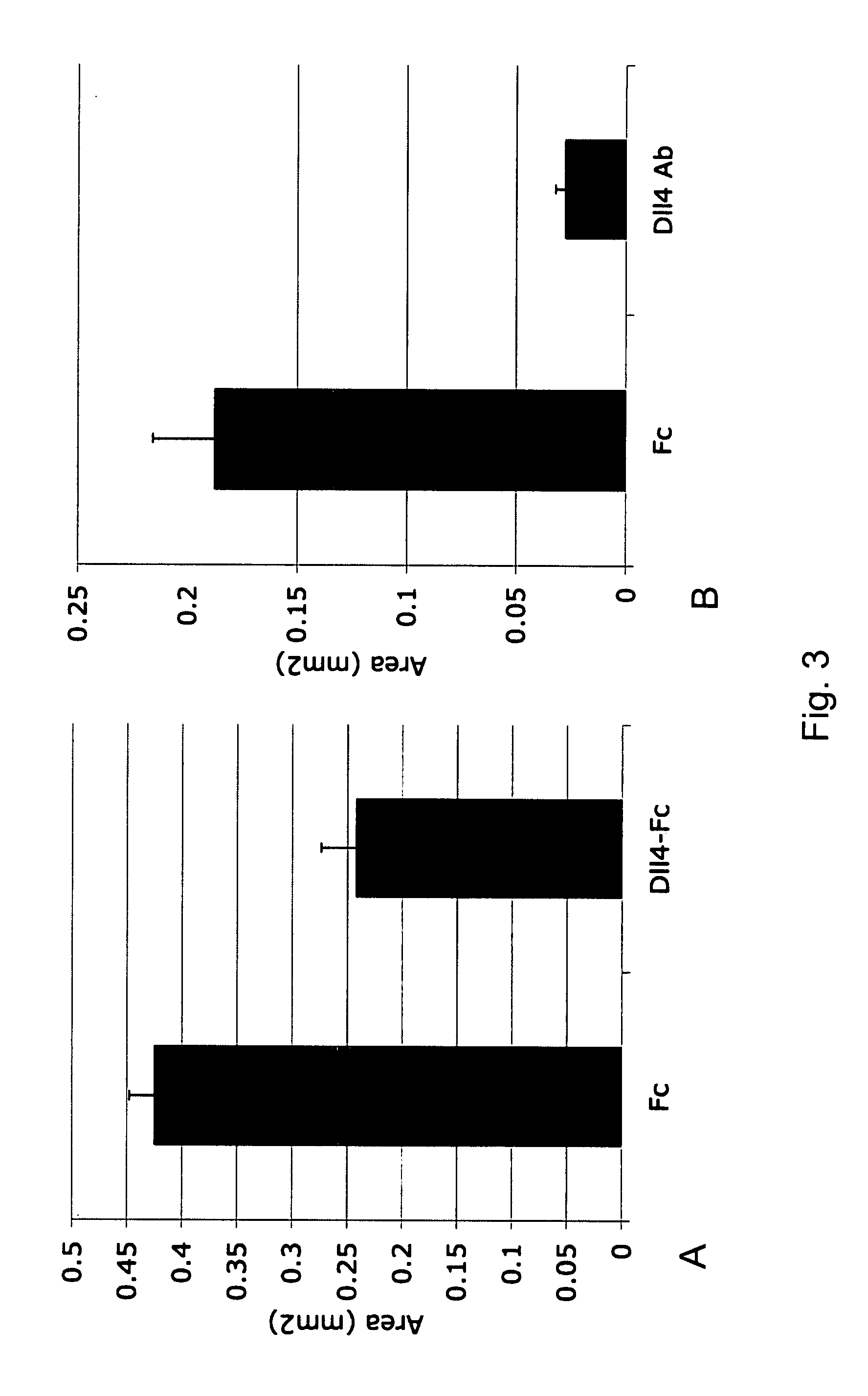
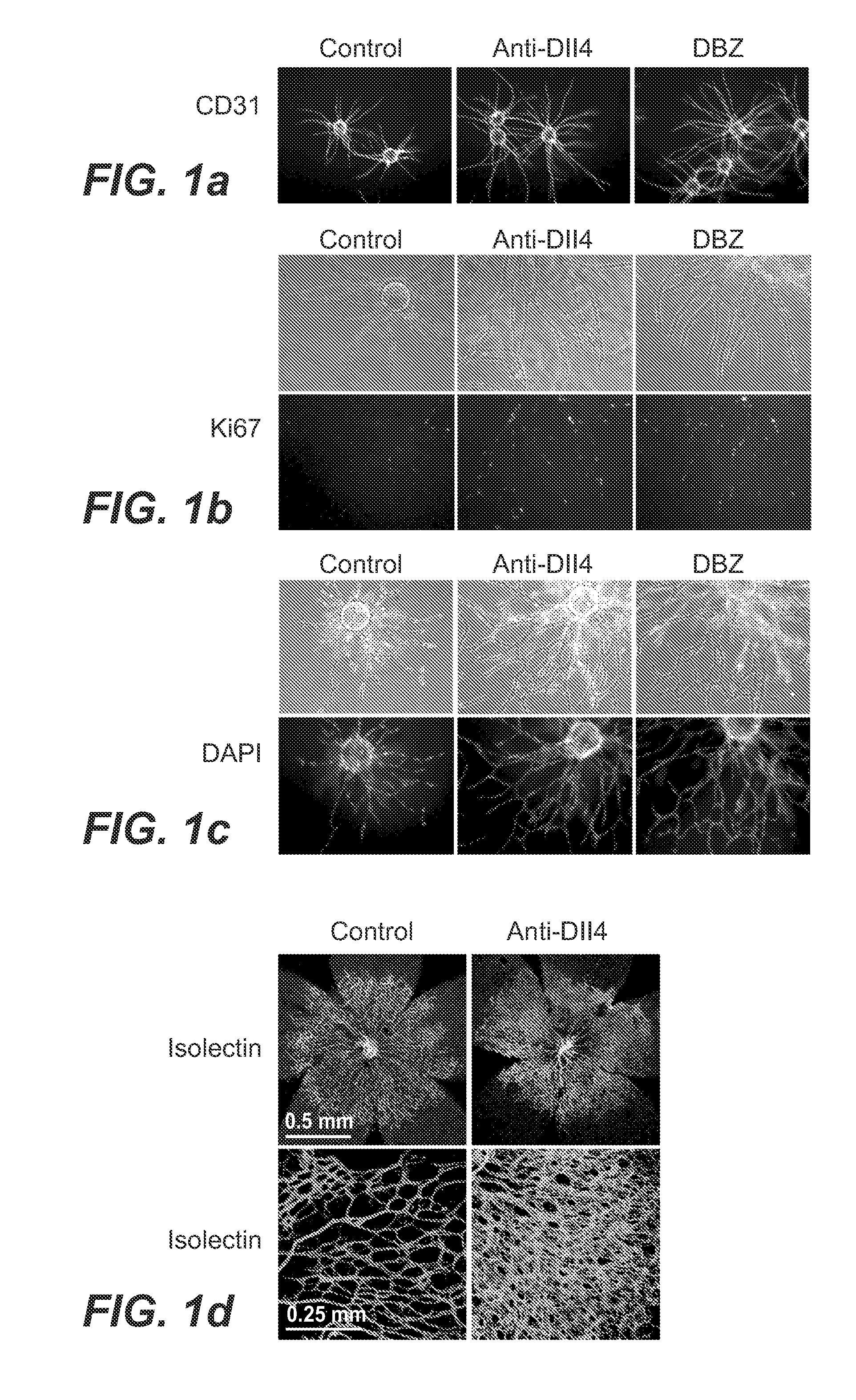
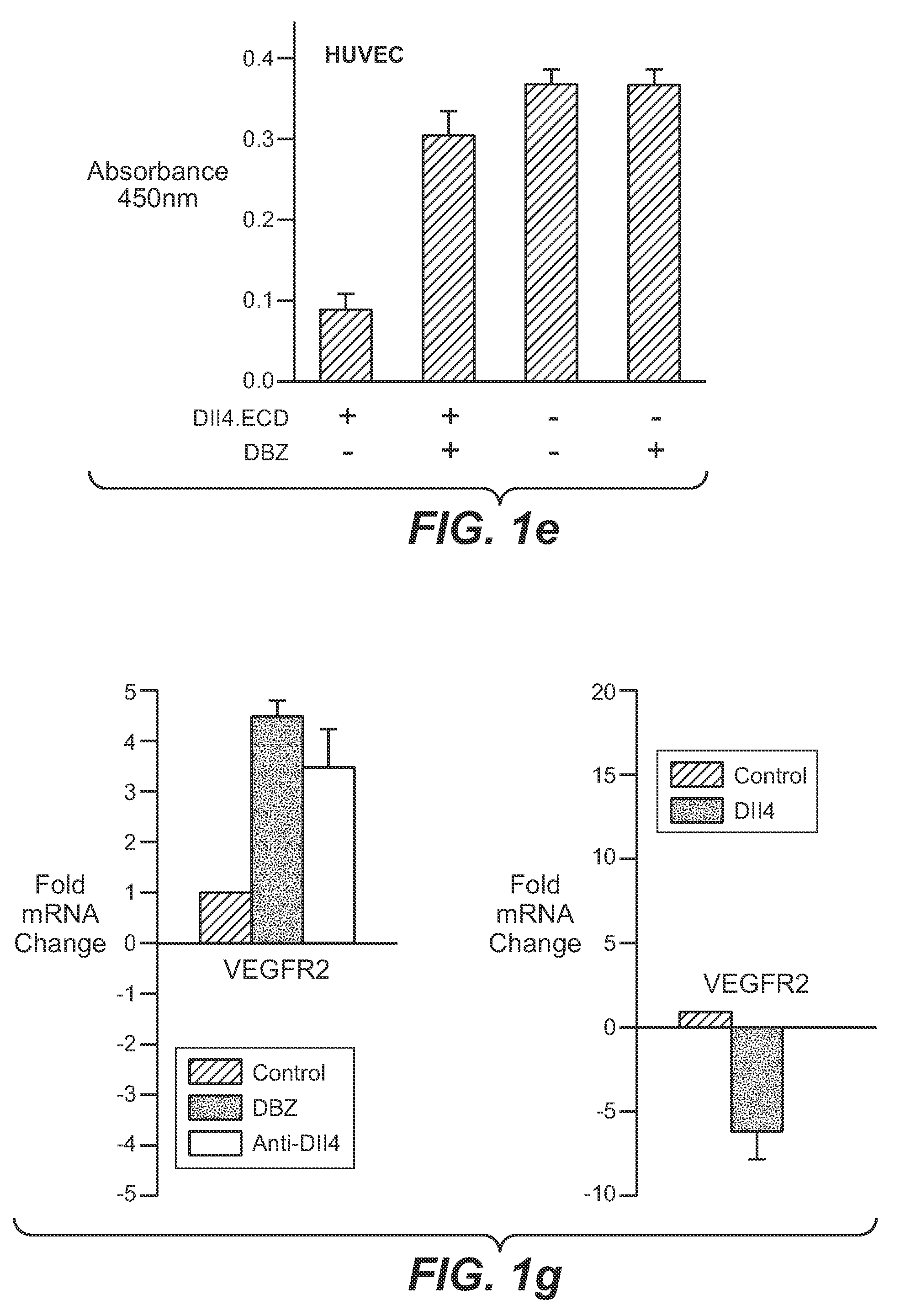
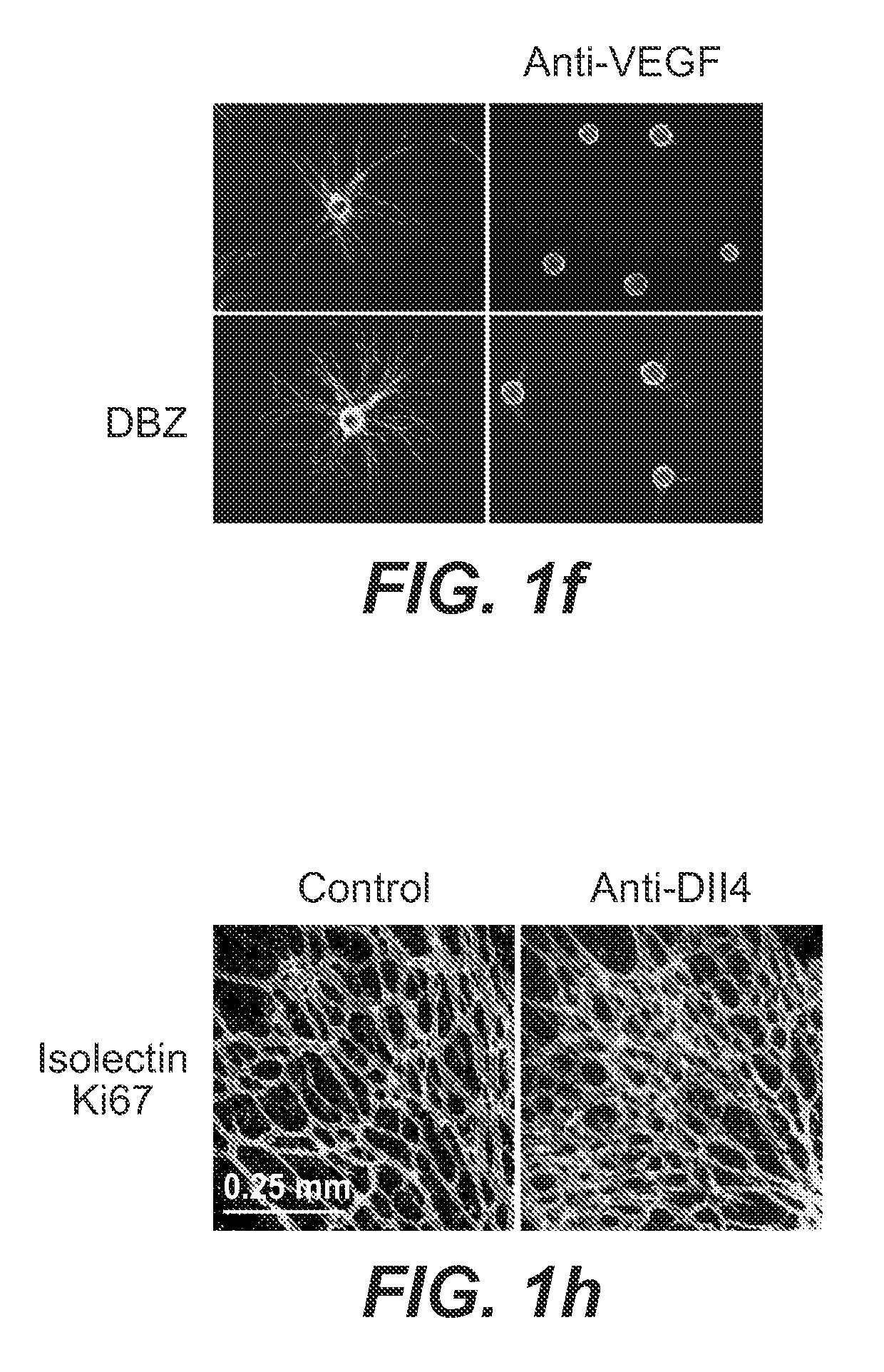
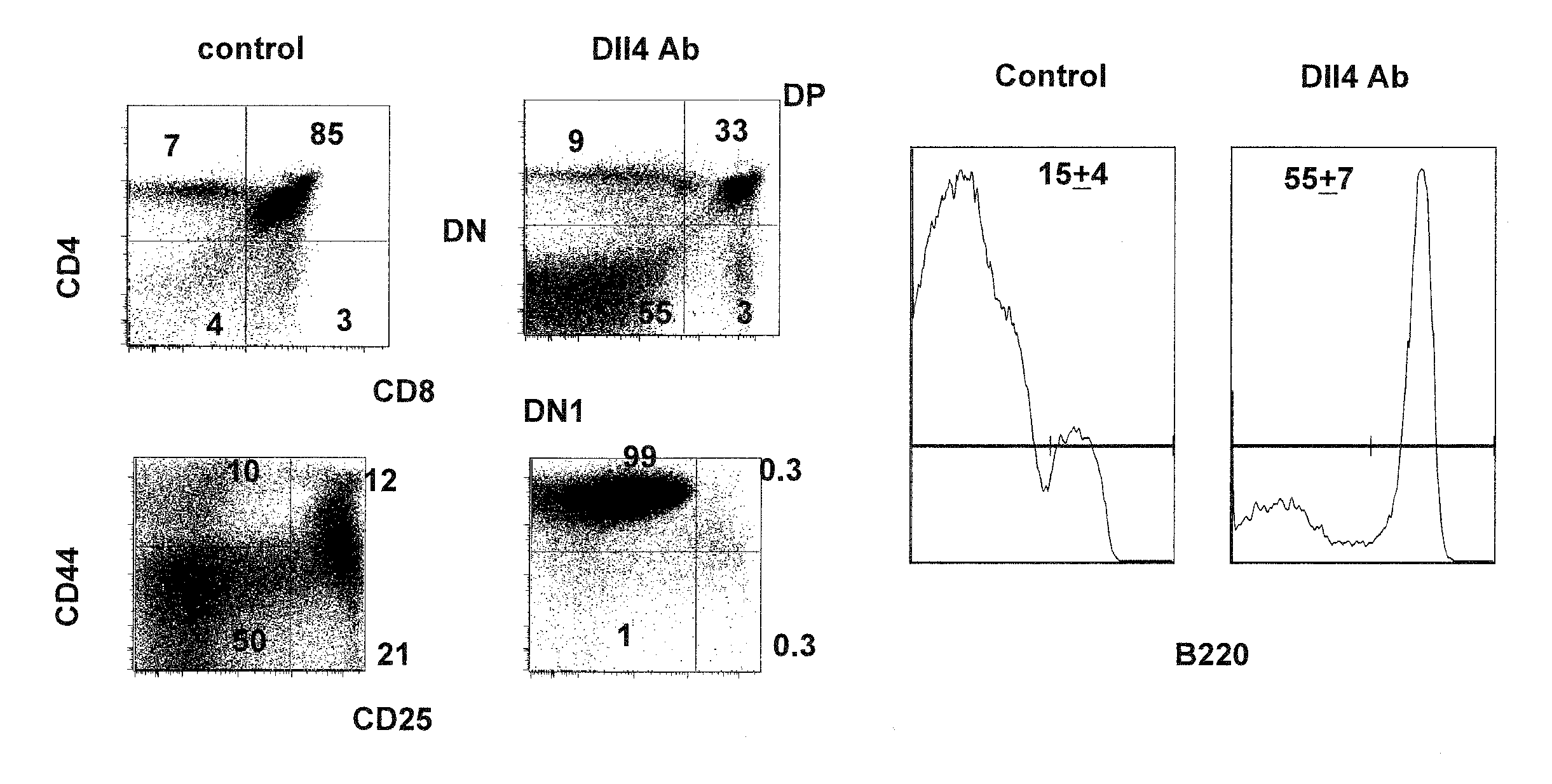
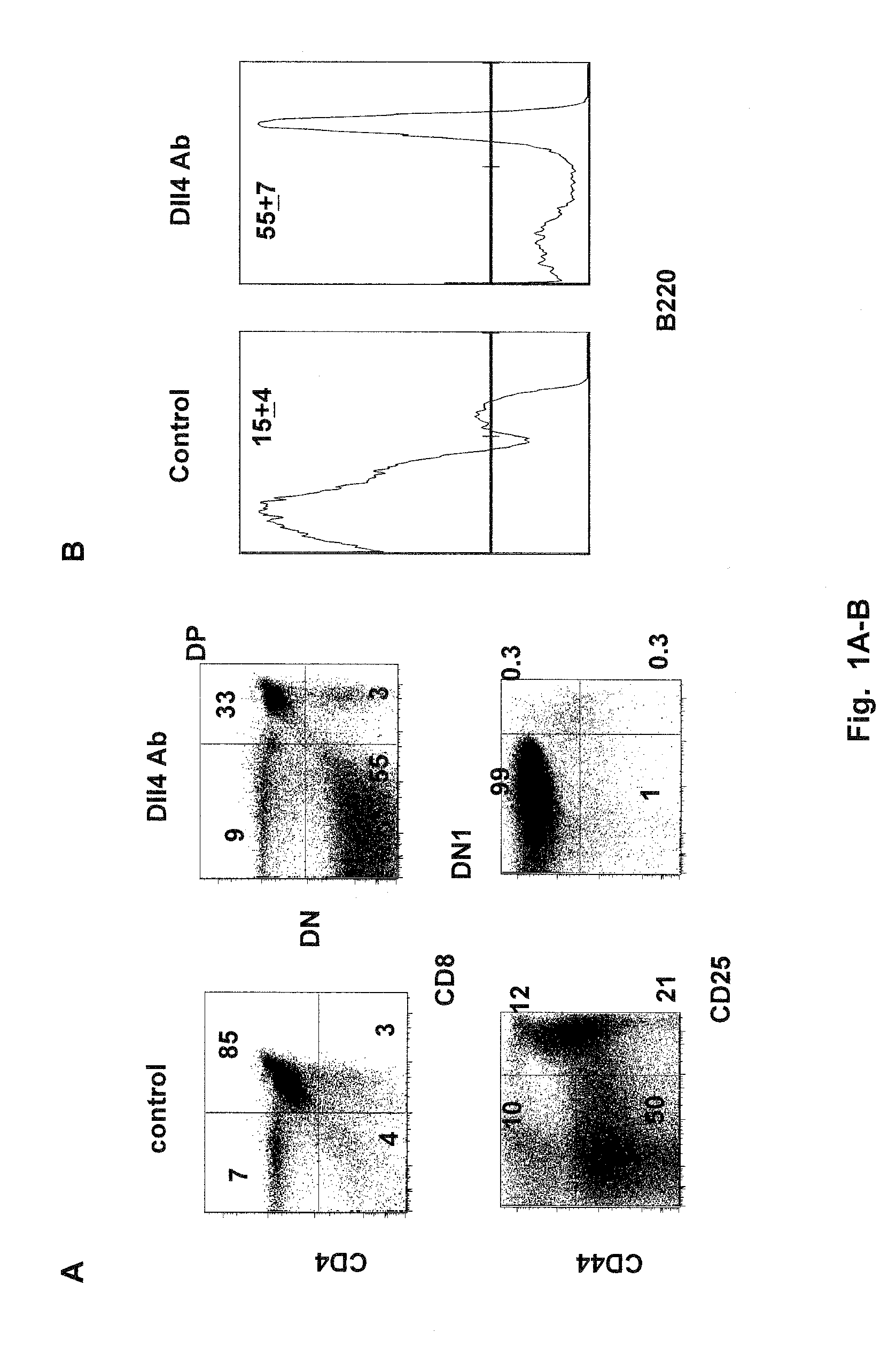
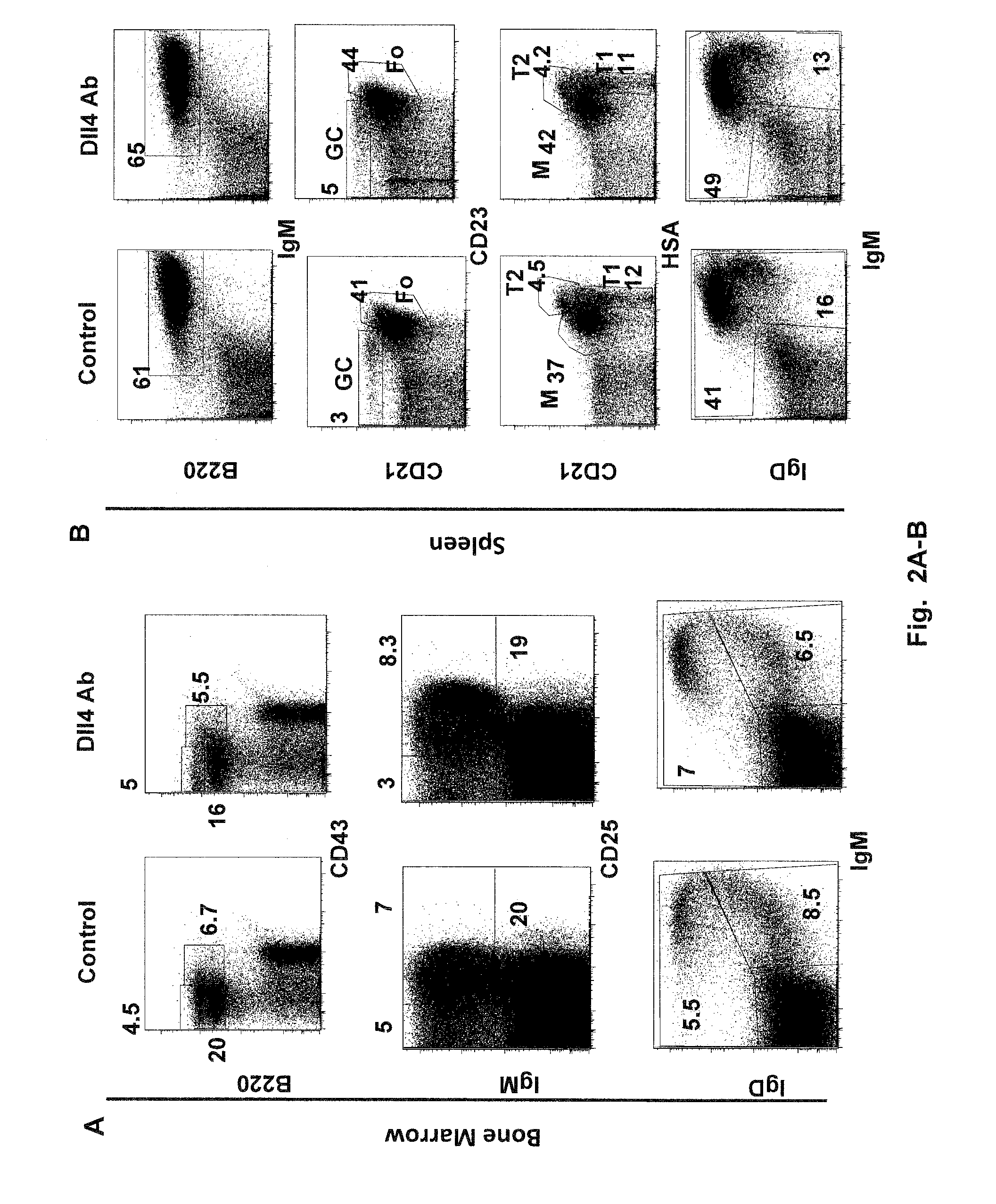
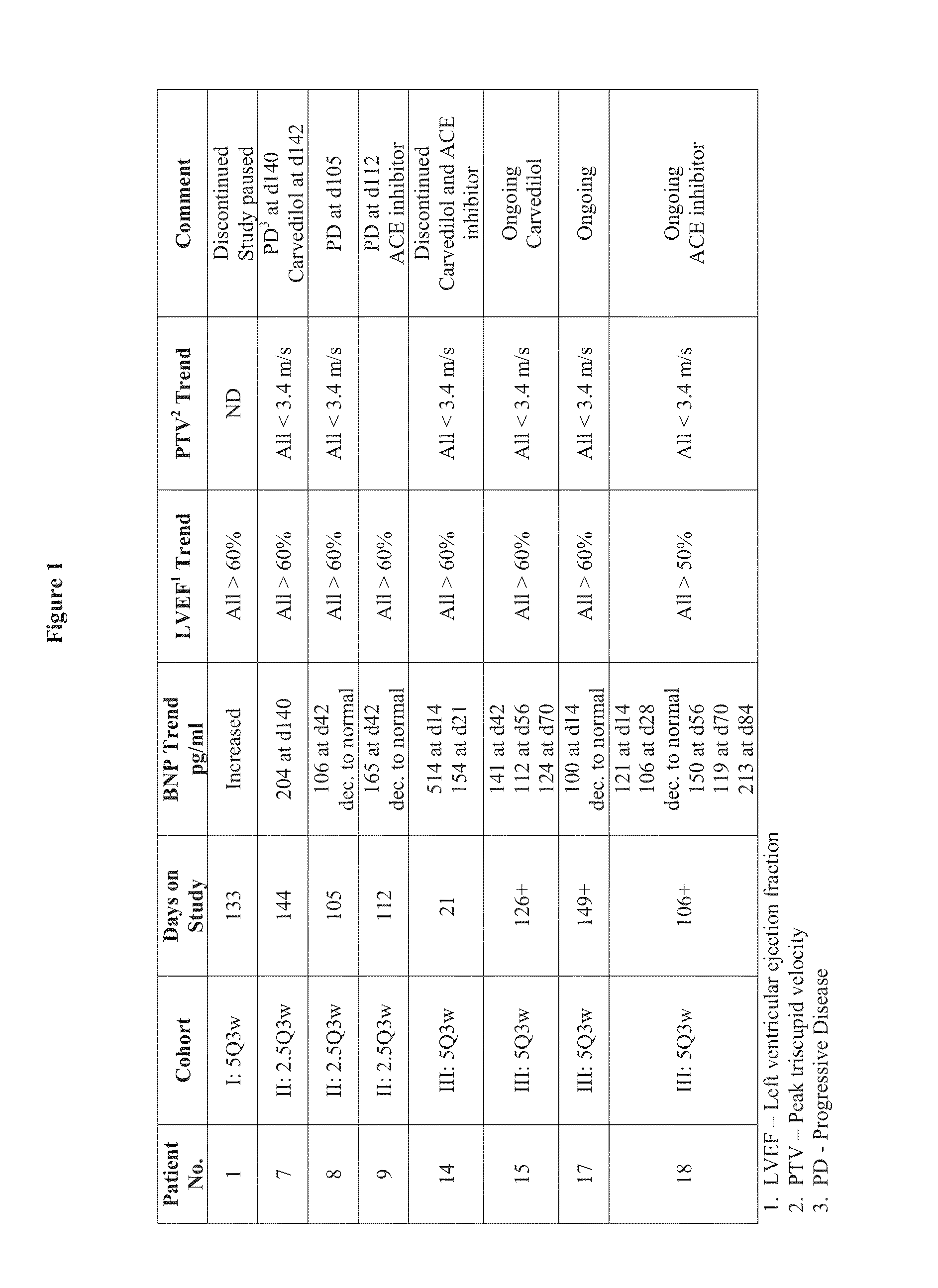
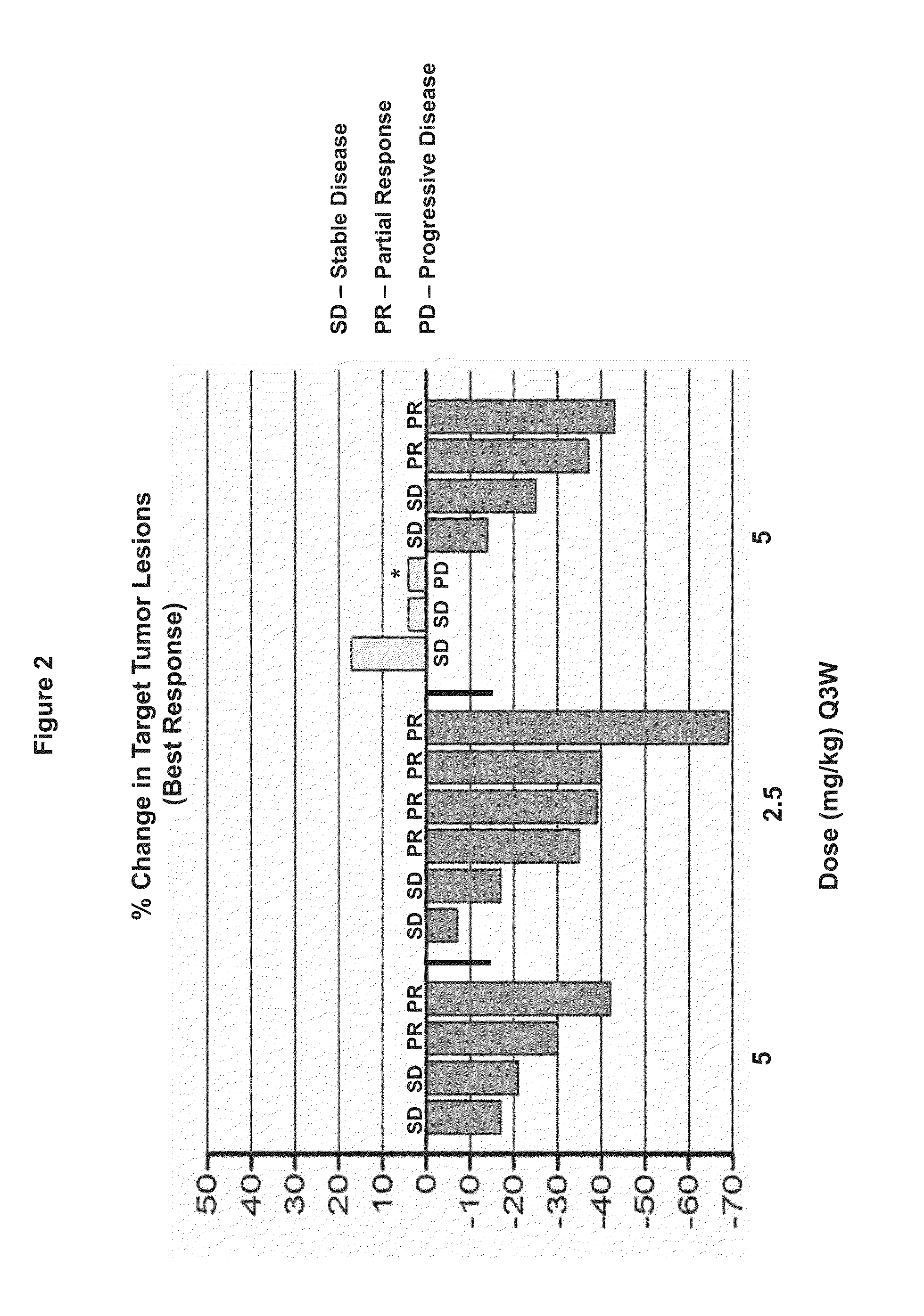
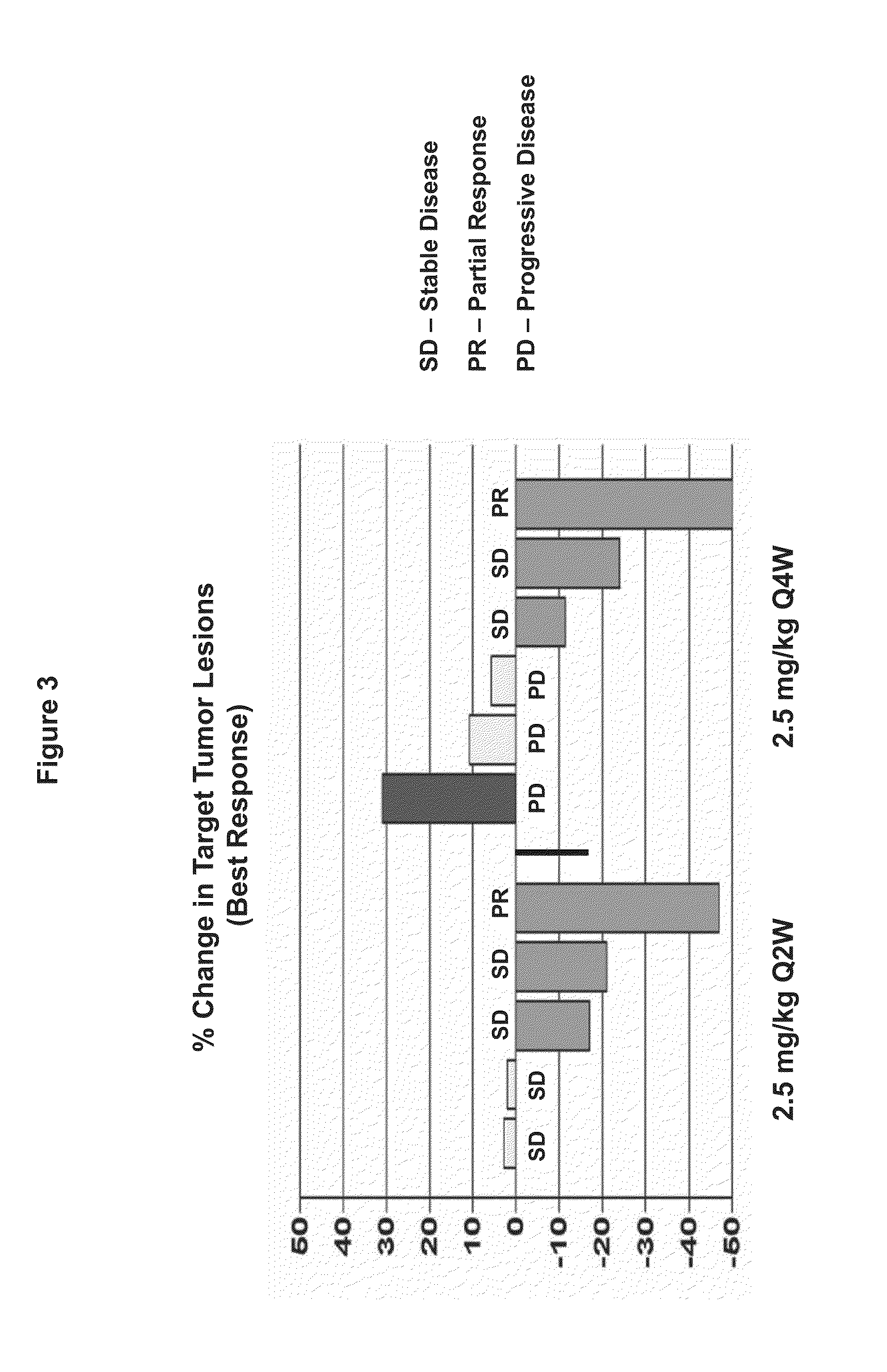
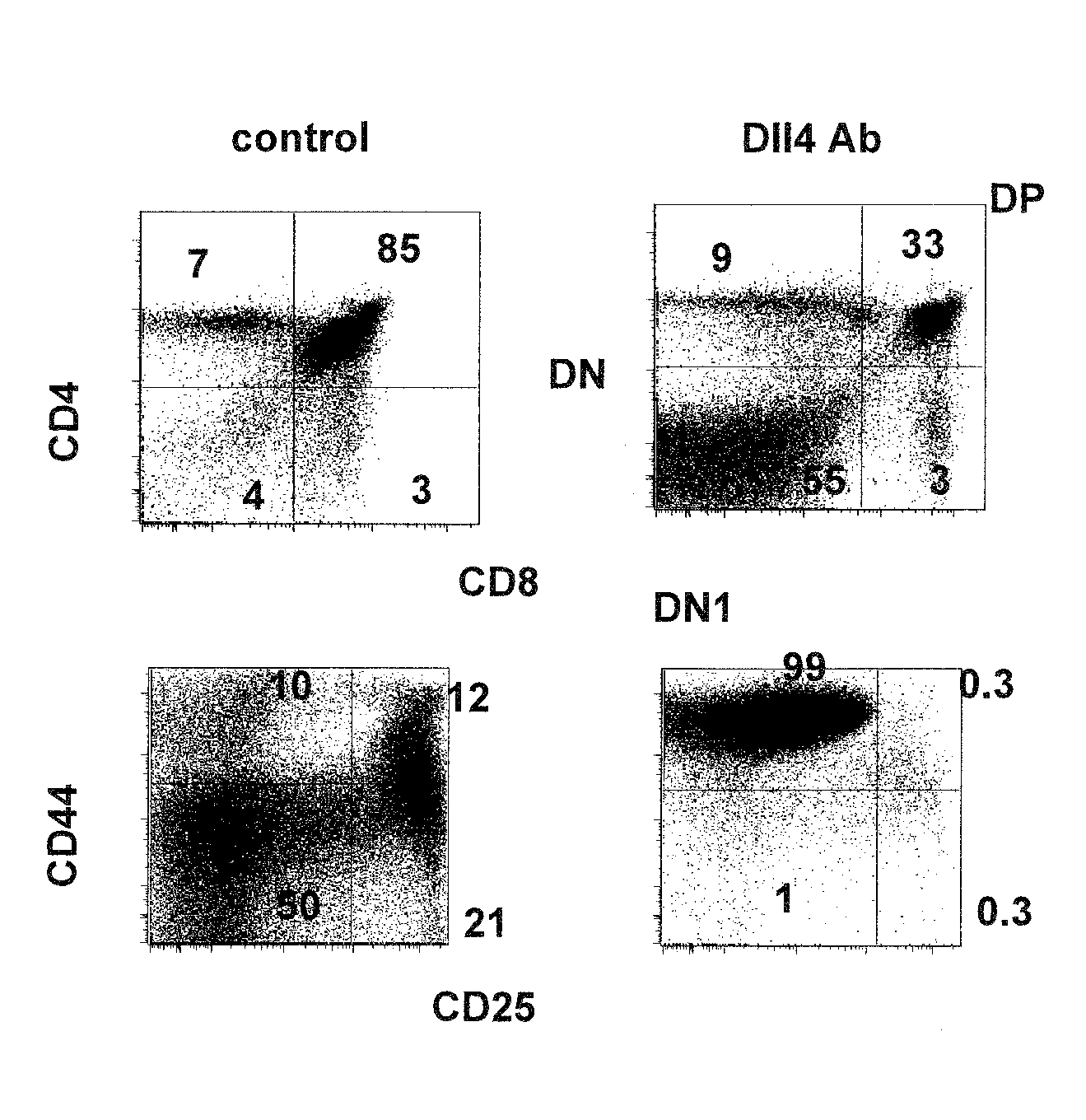
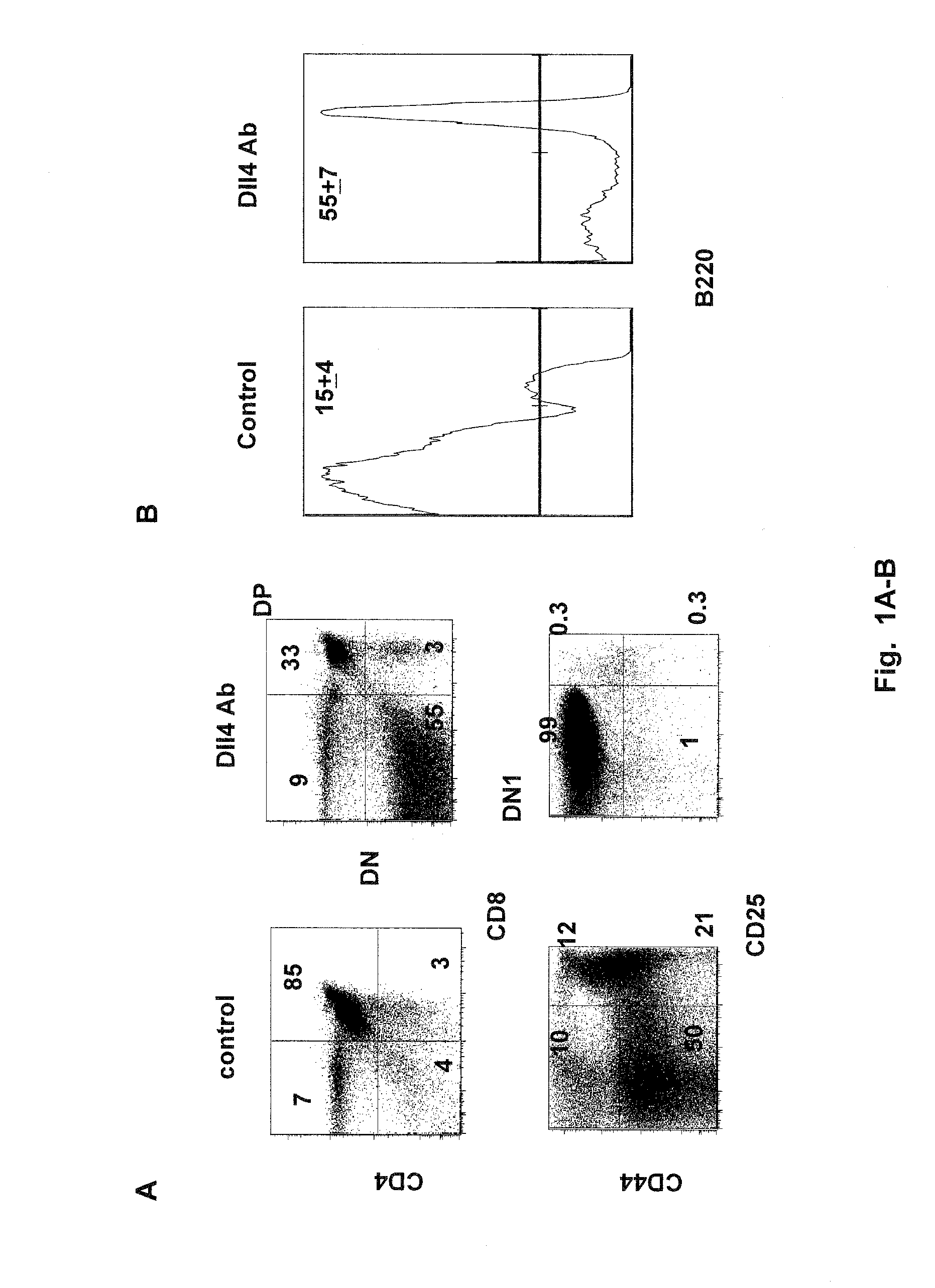
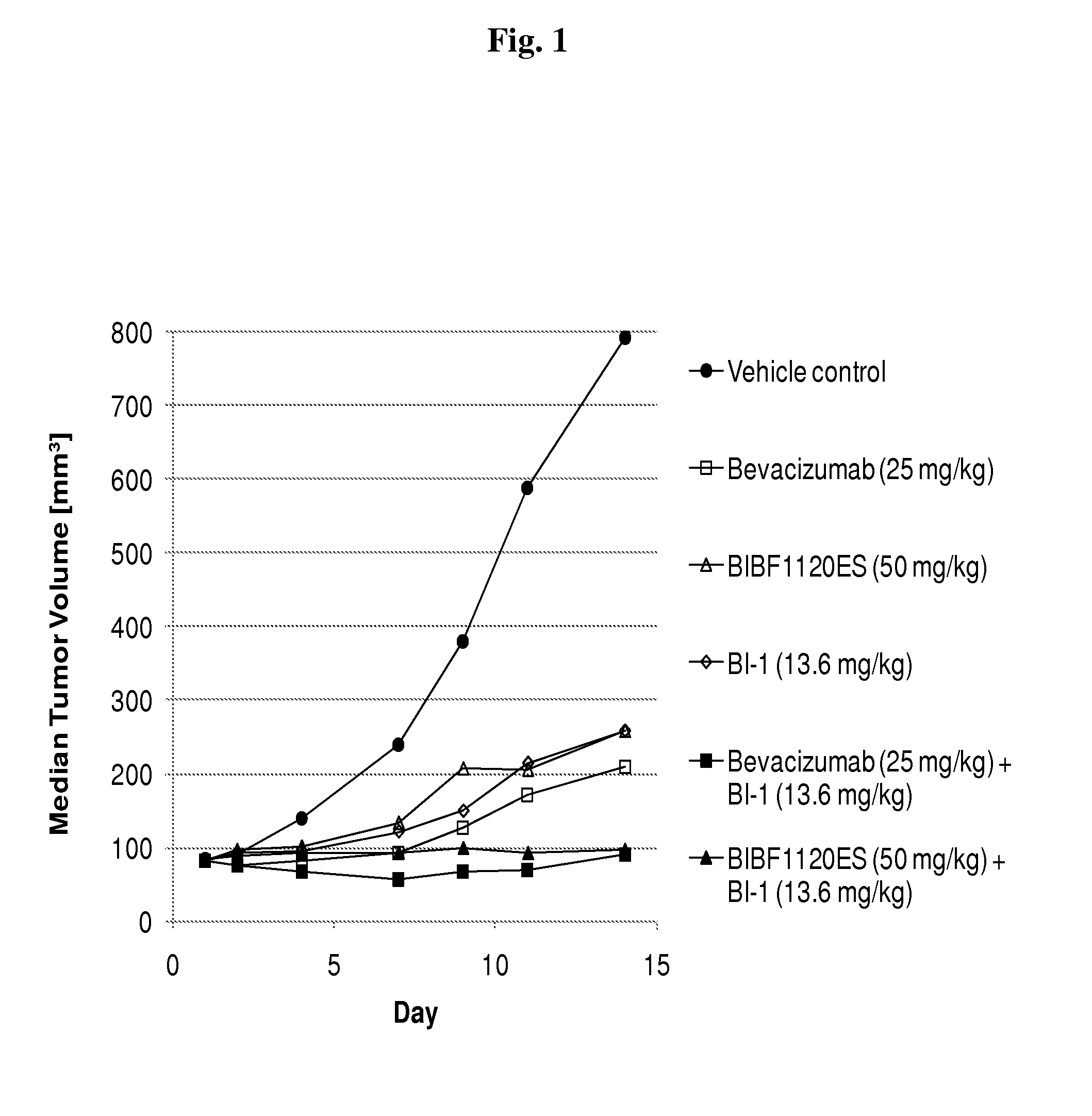
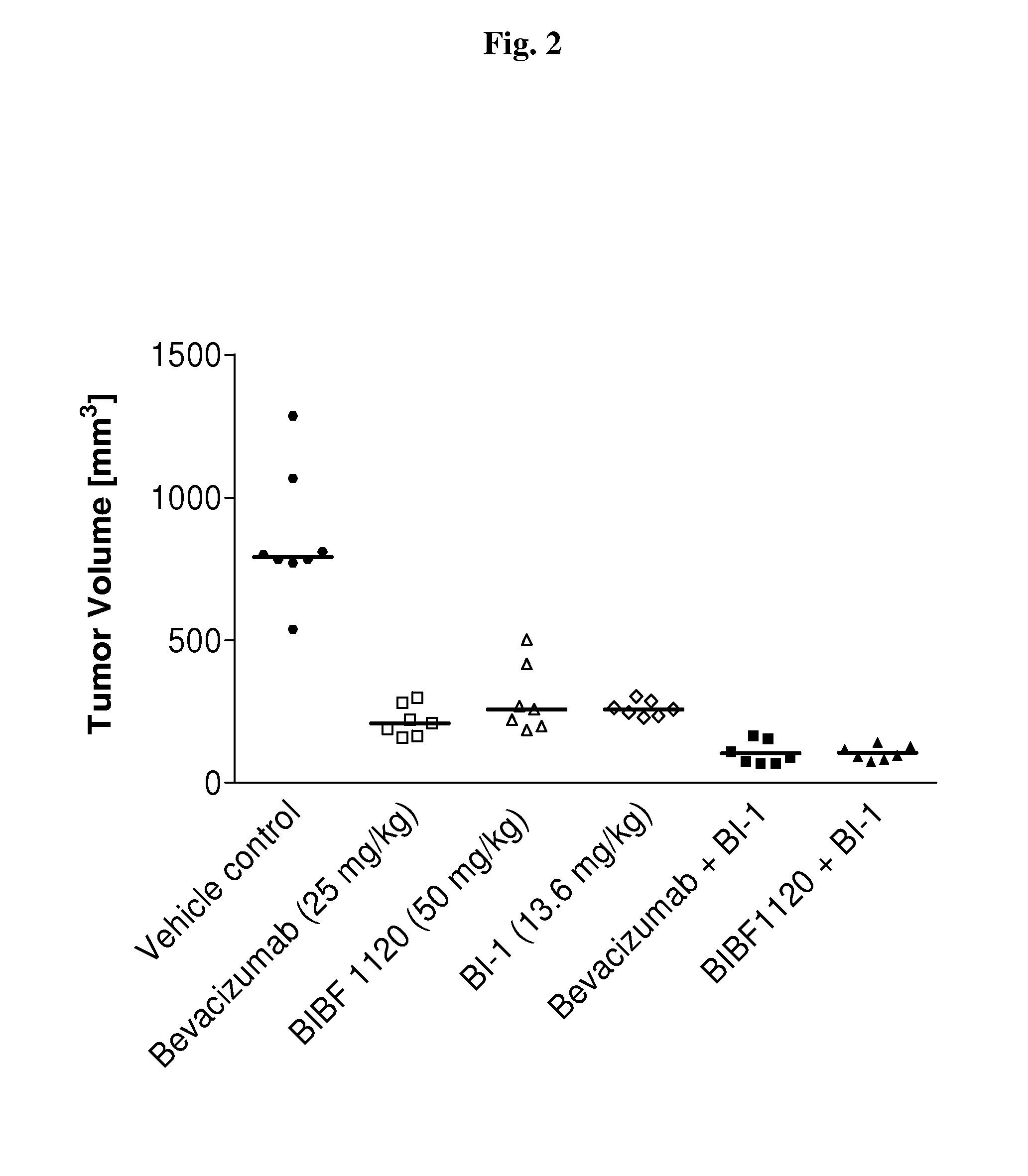
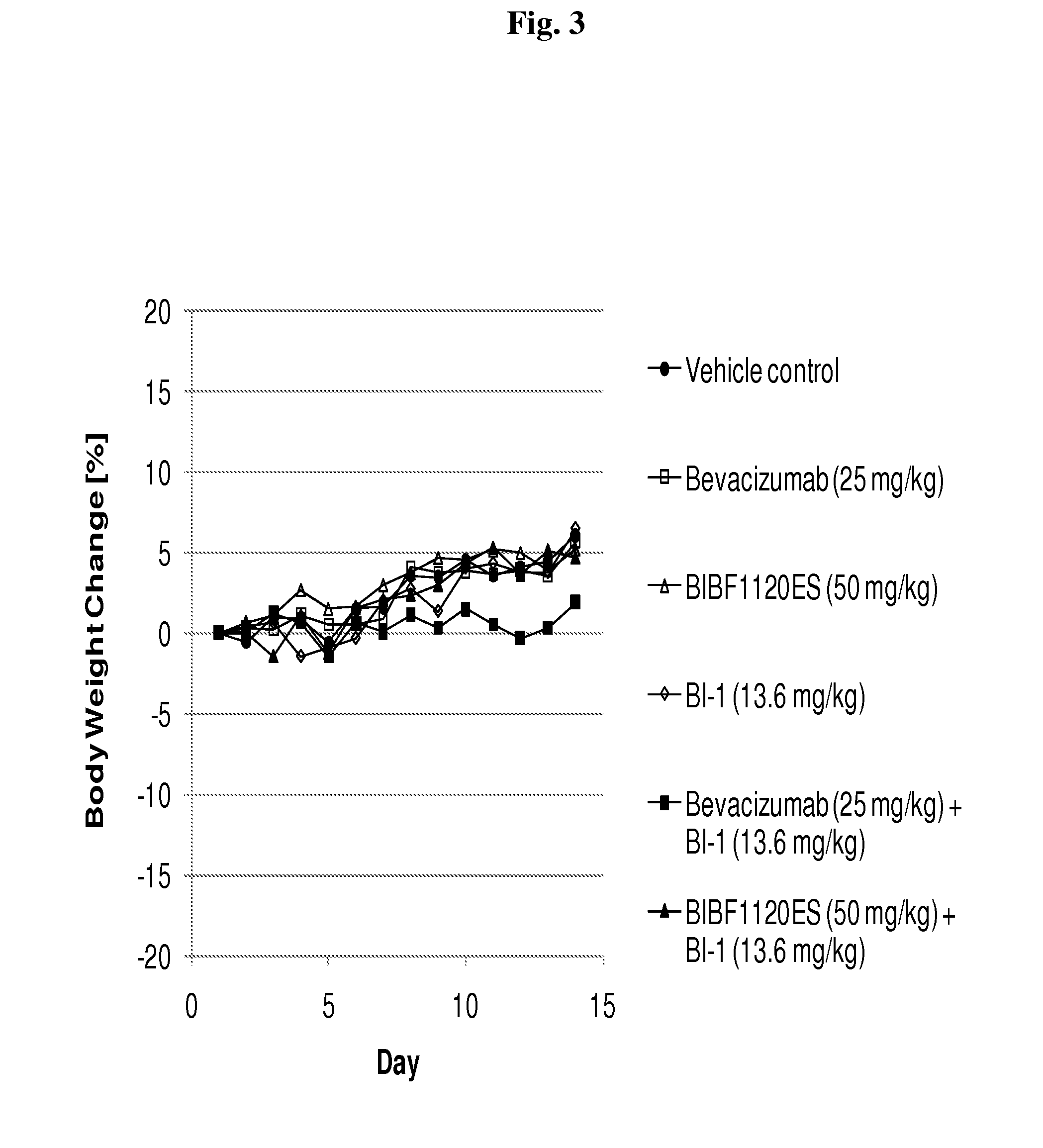
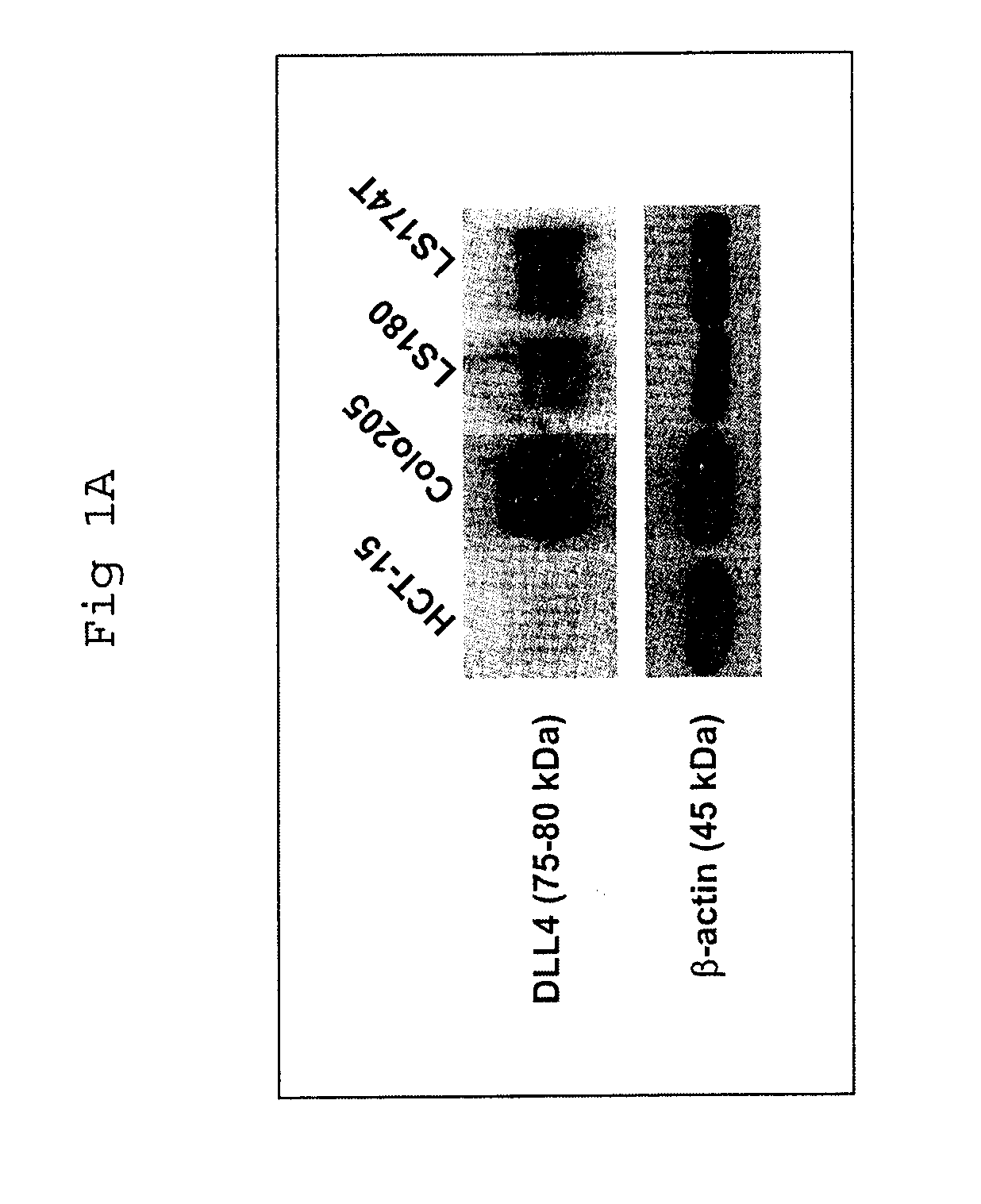
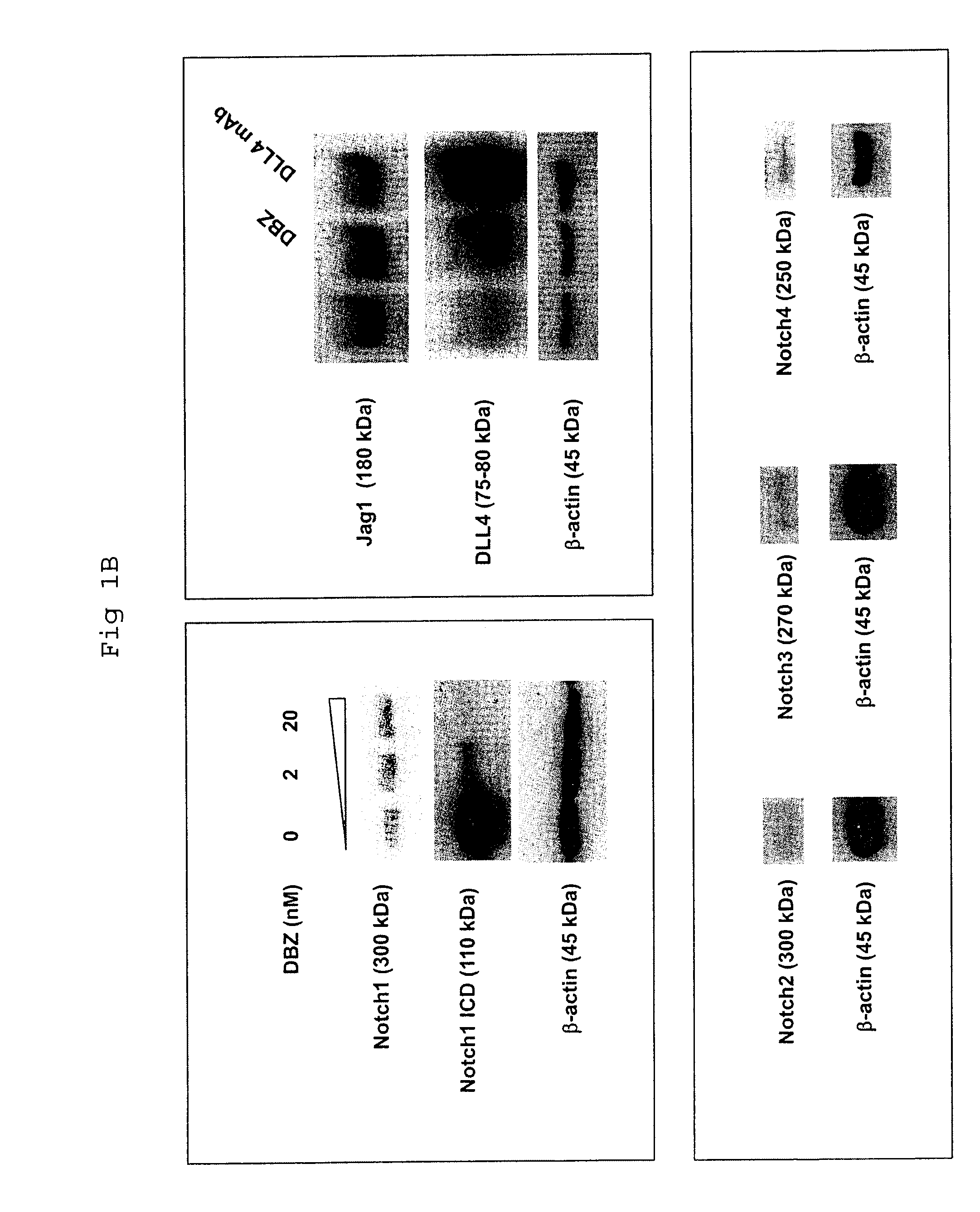
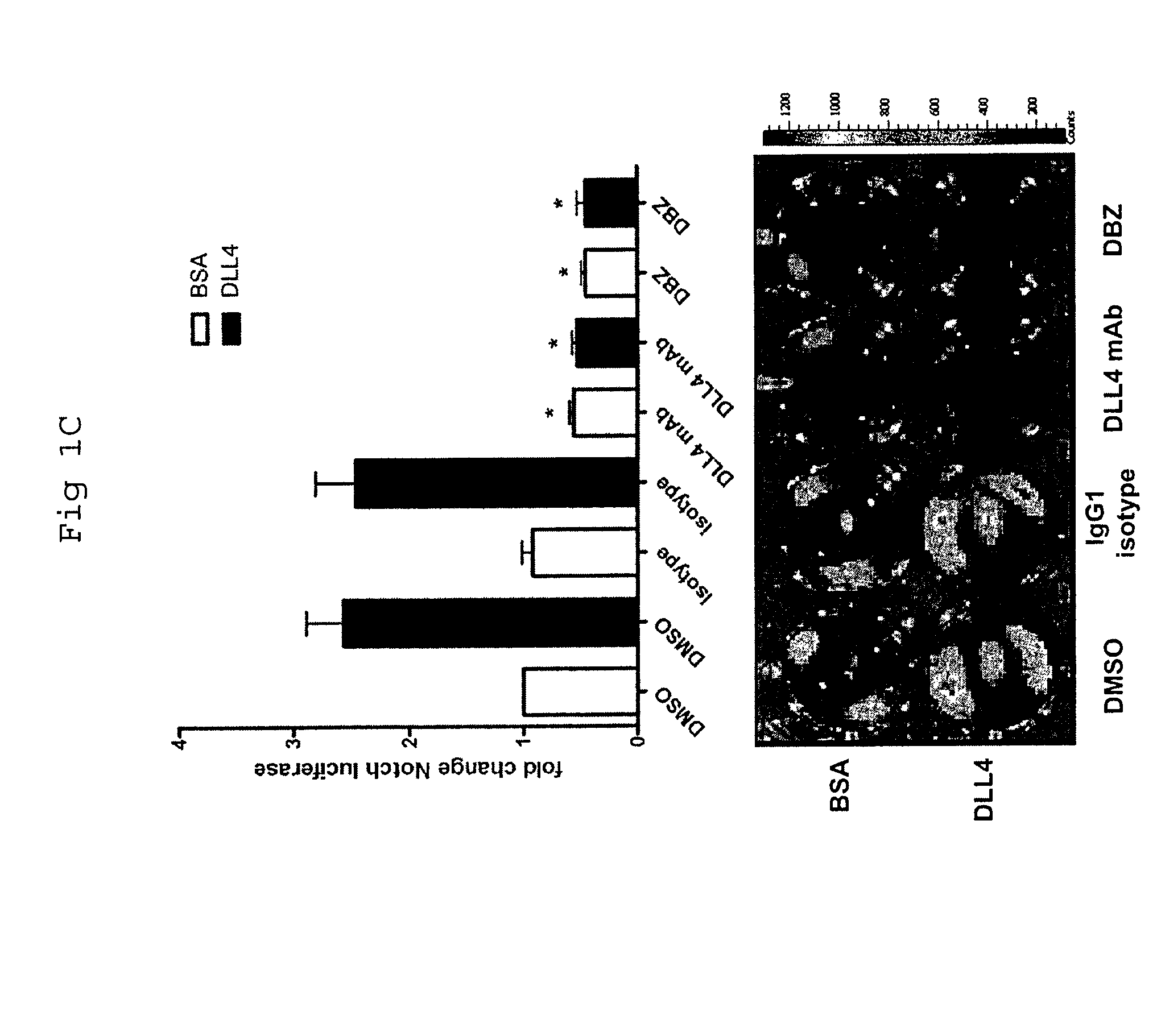
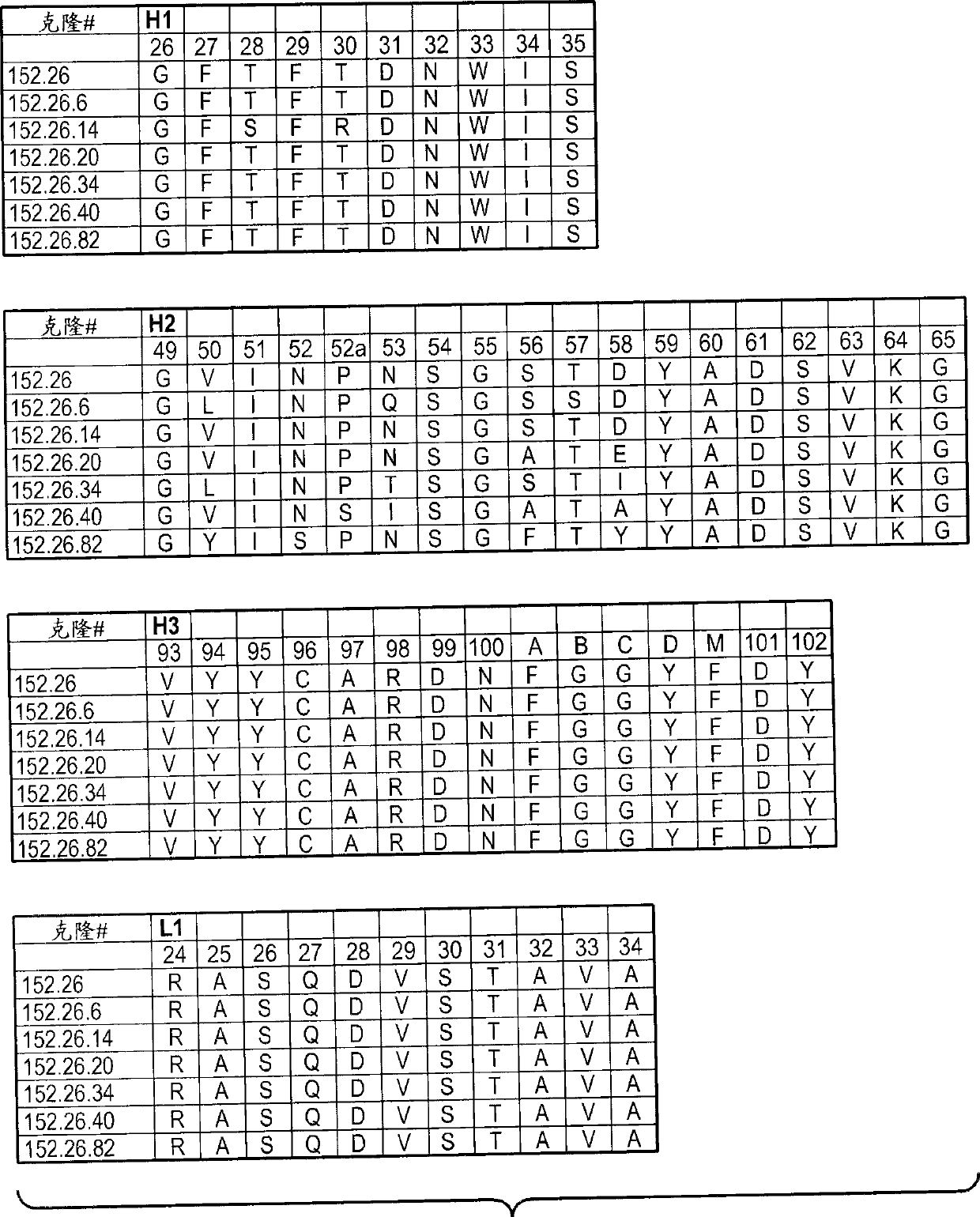
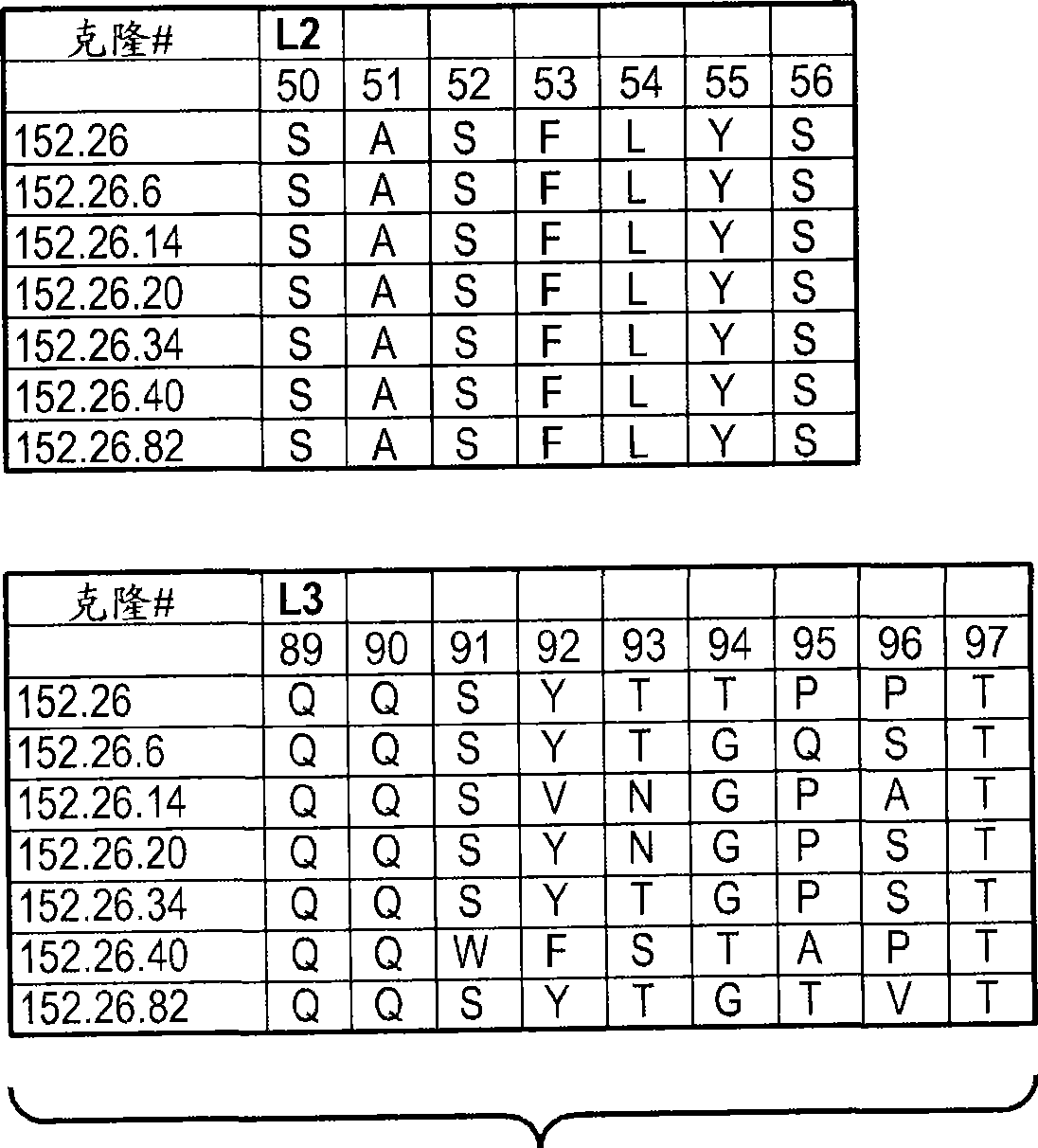
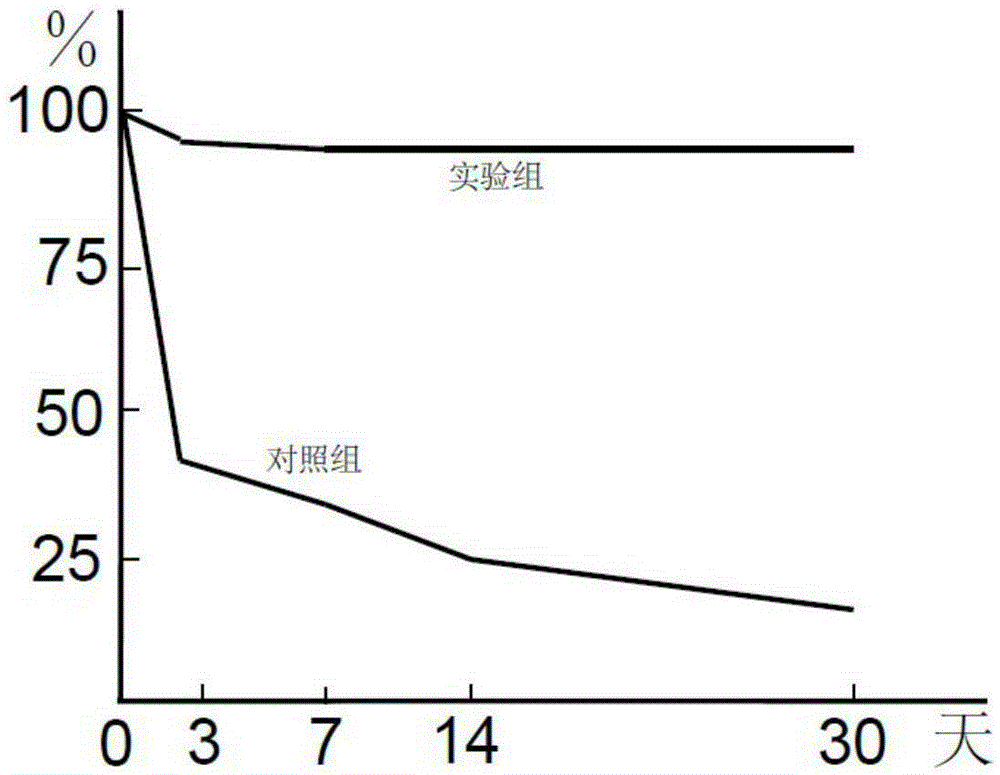
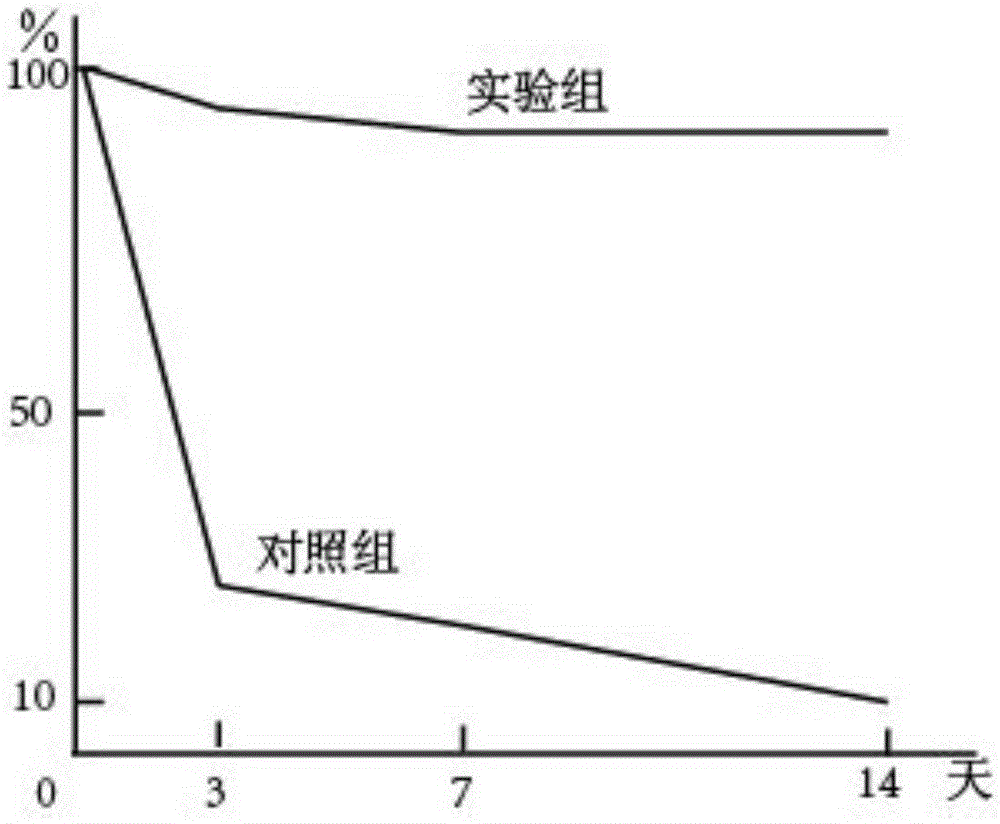
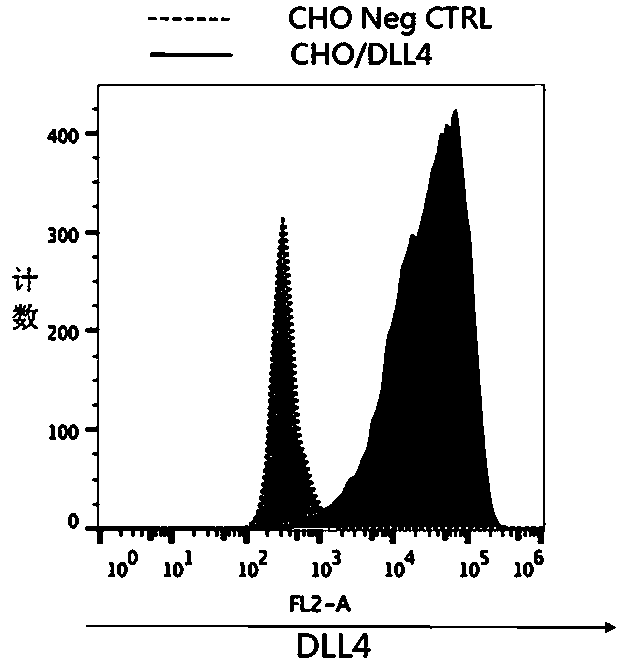
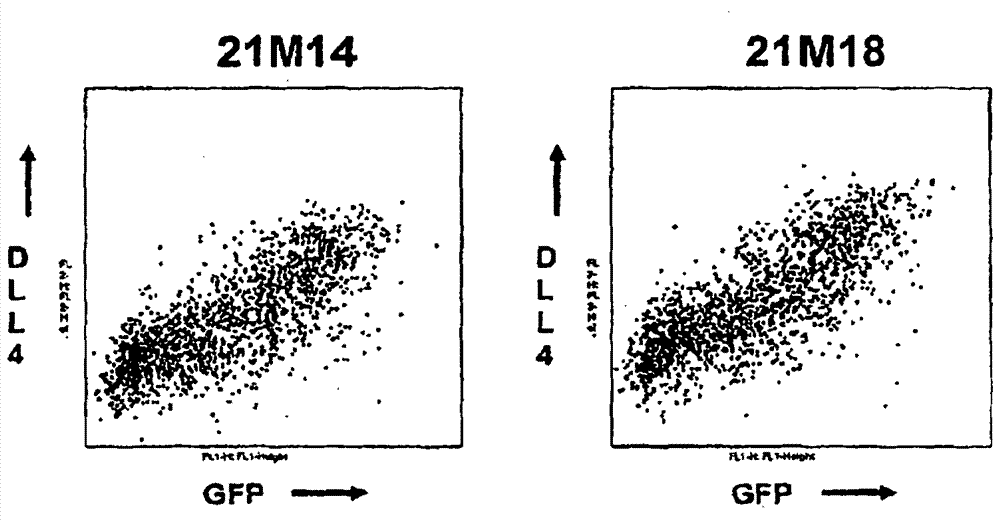
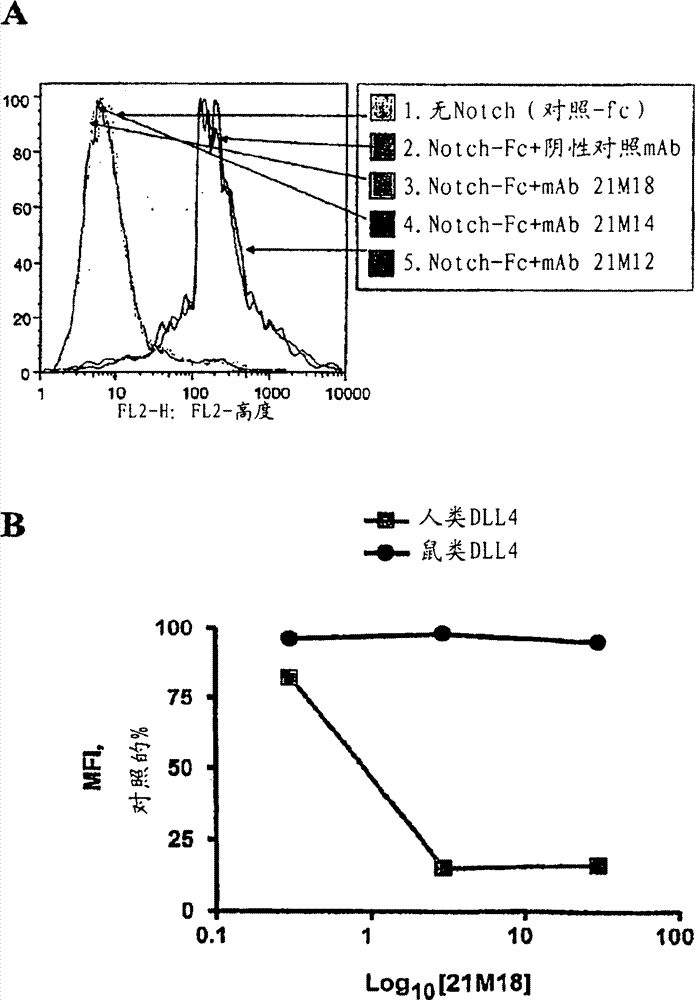
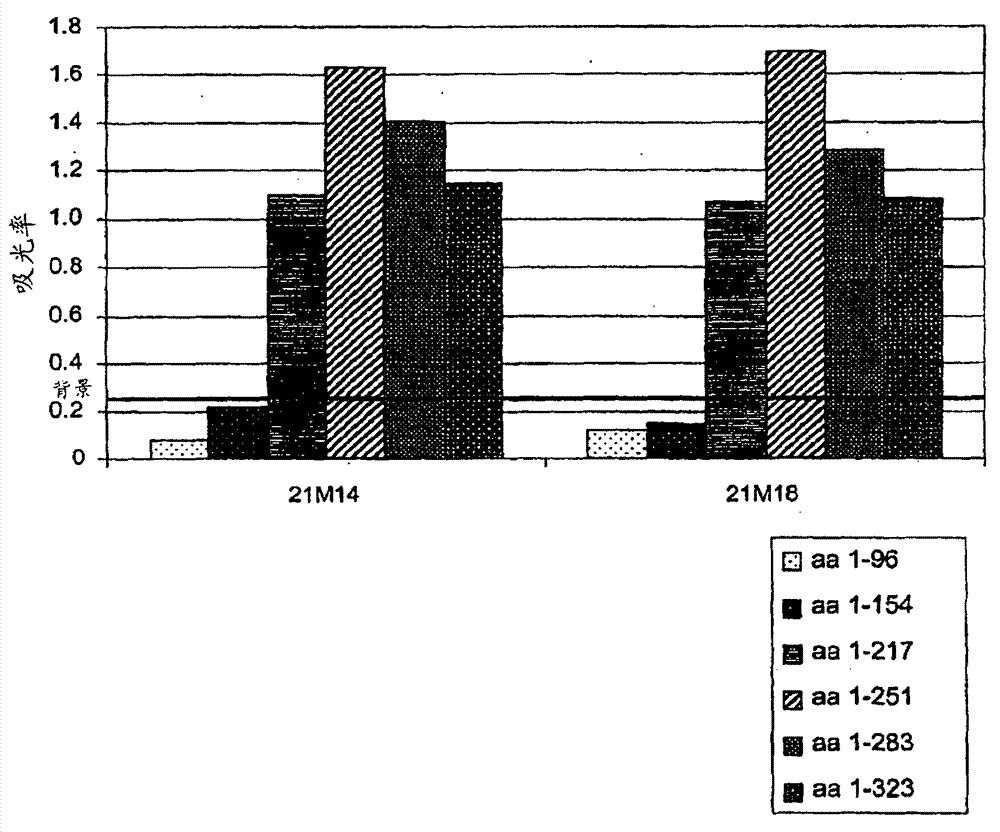
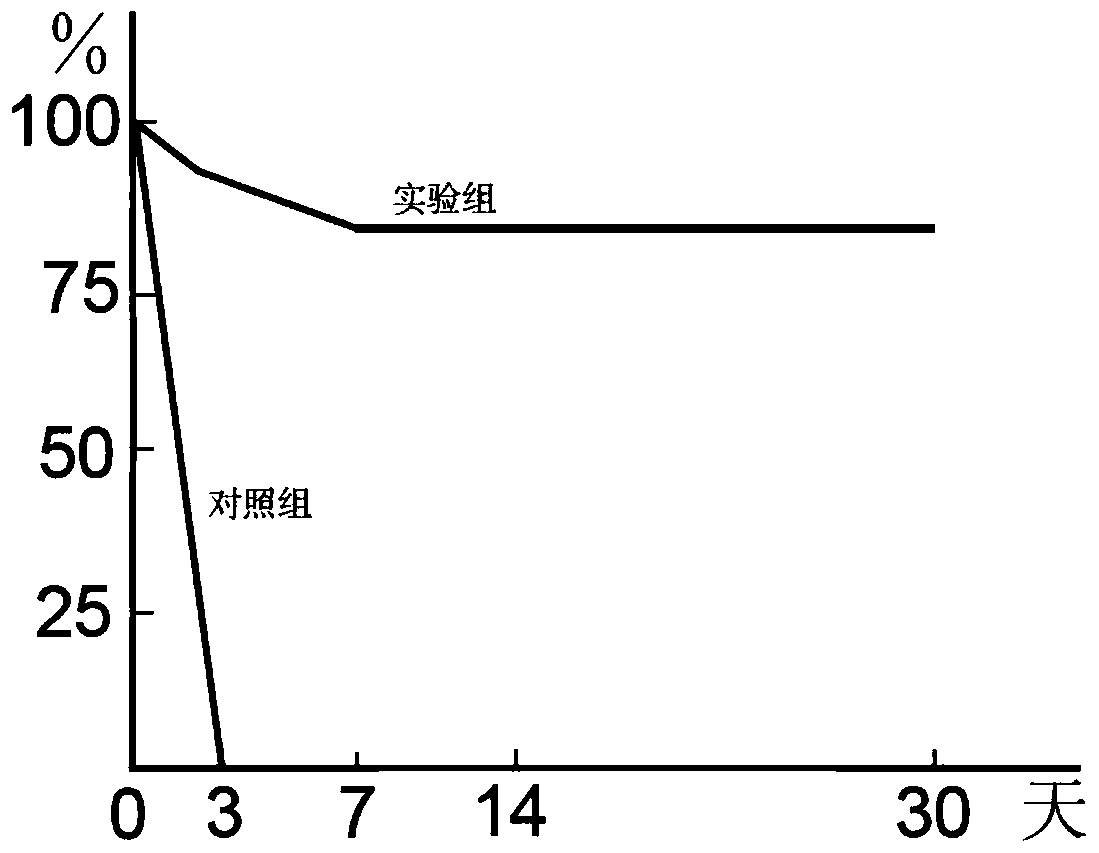
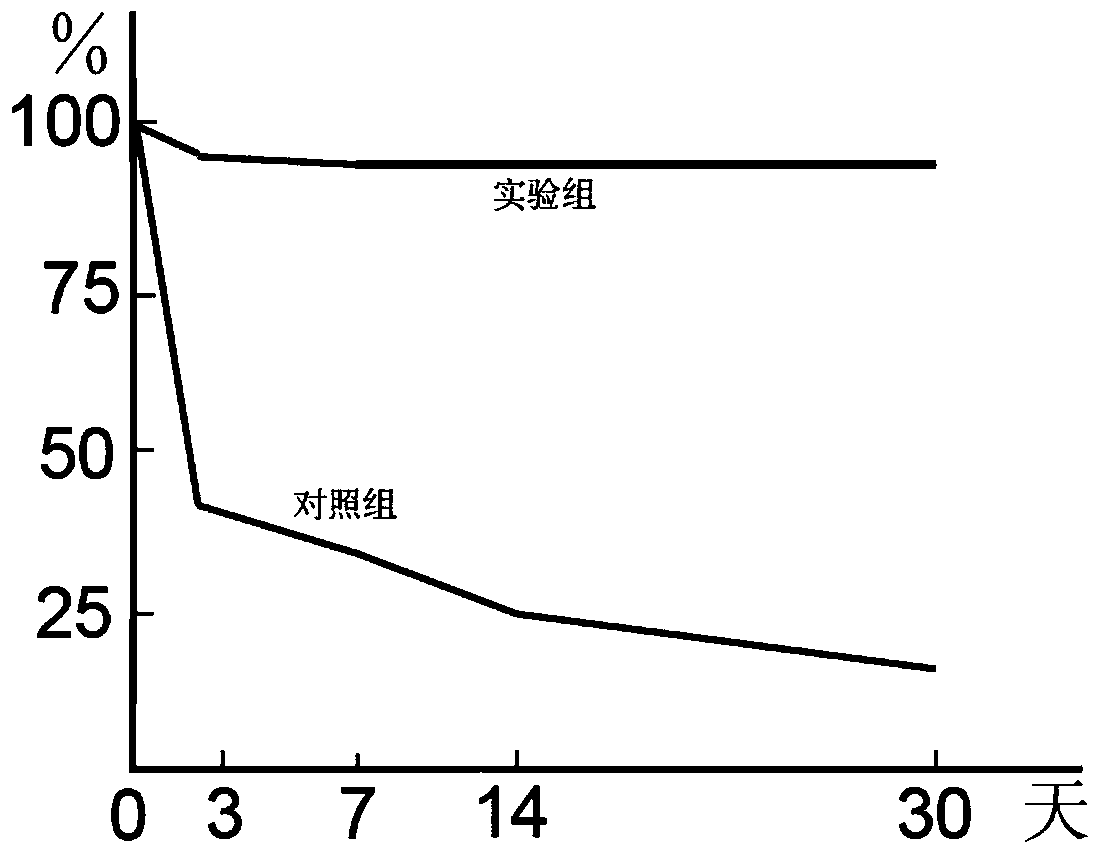
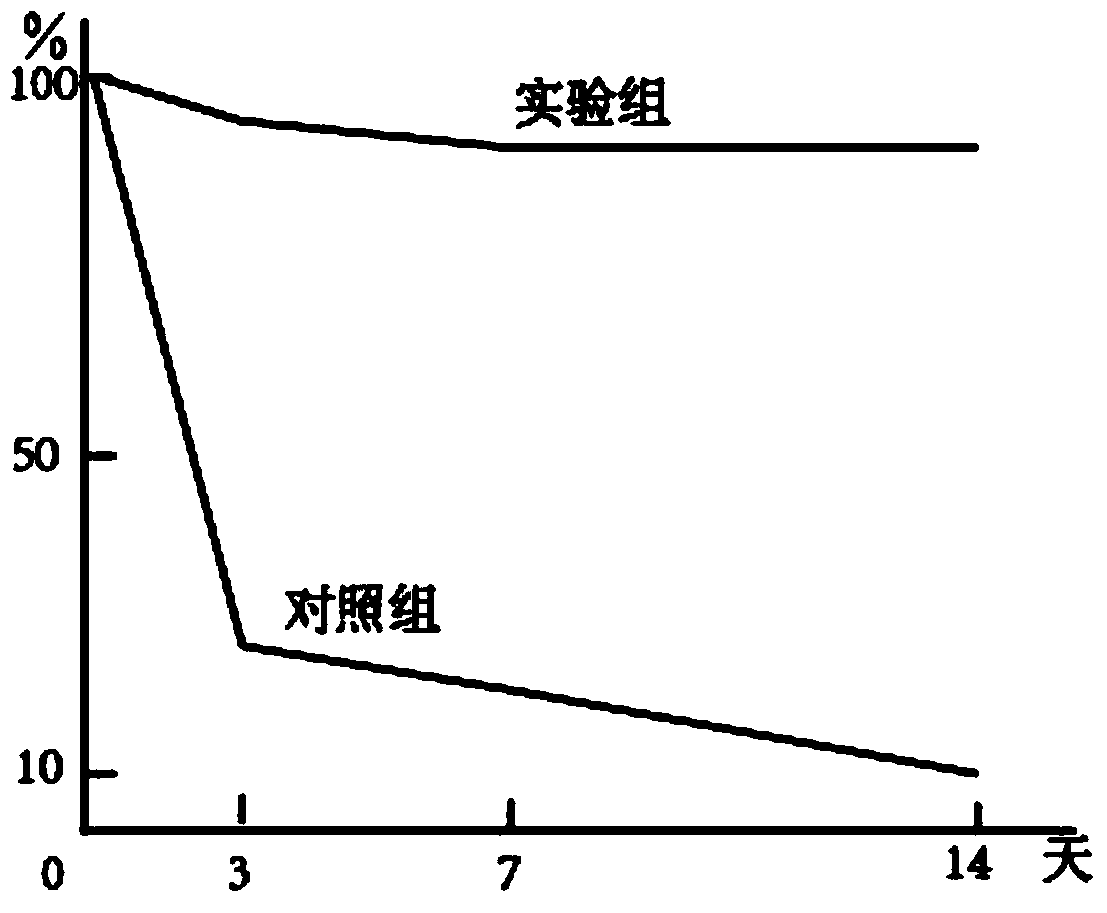
Patents
Literature
33 results about "Delta-like ligand 4" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
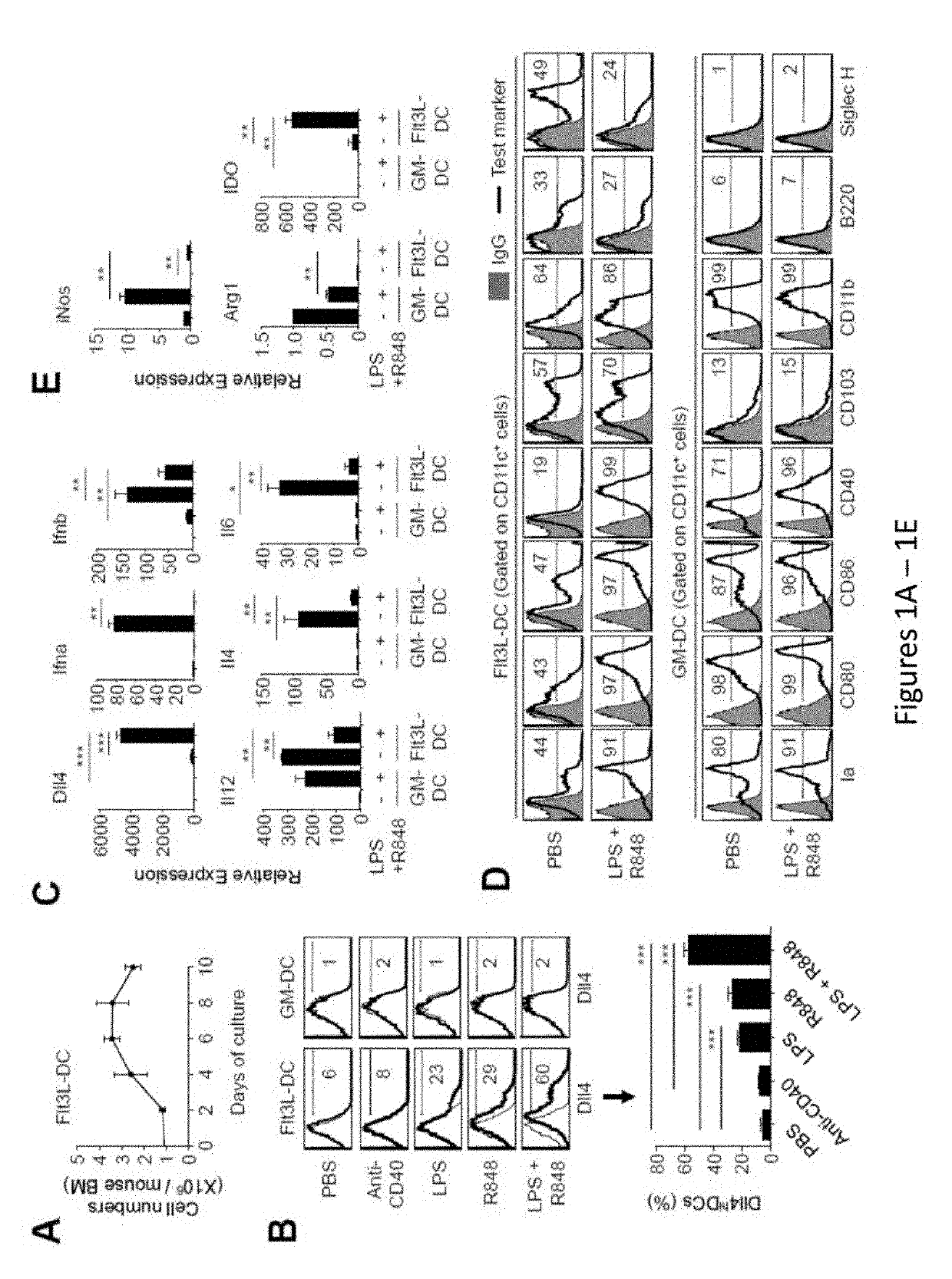
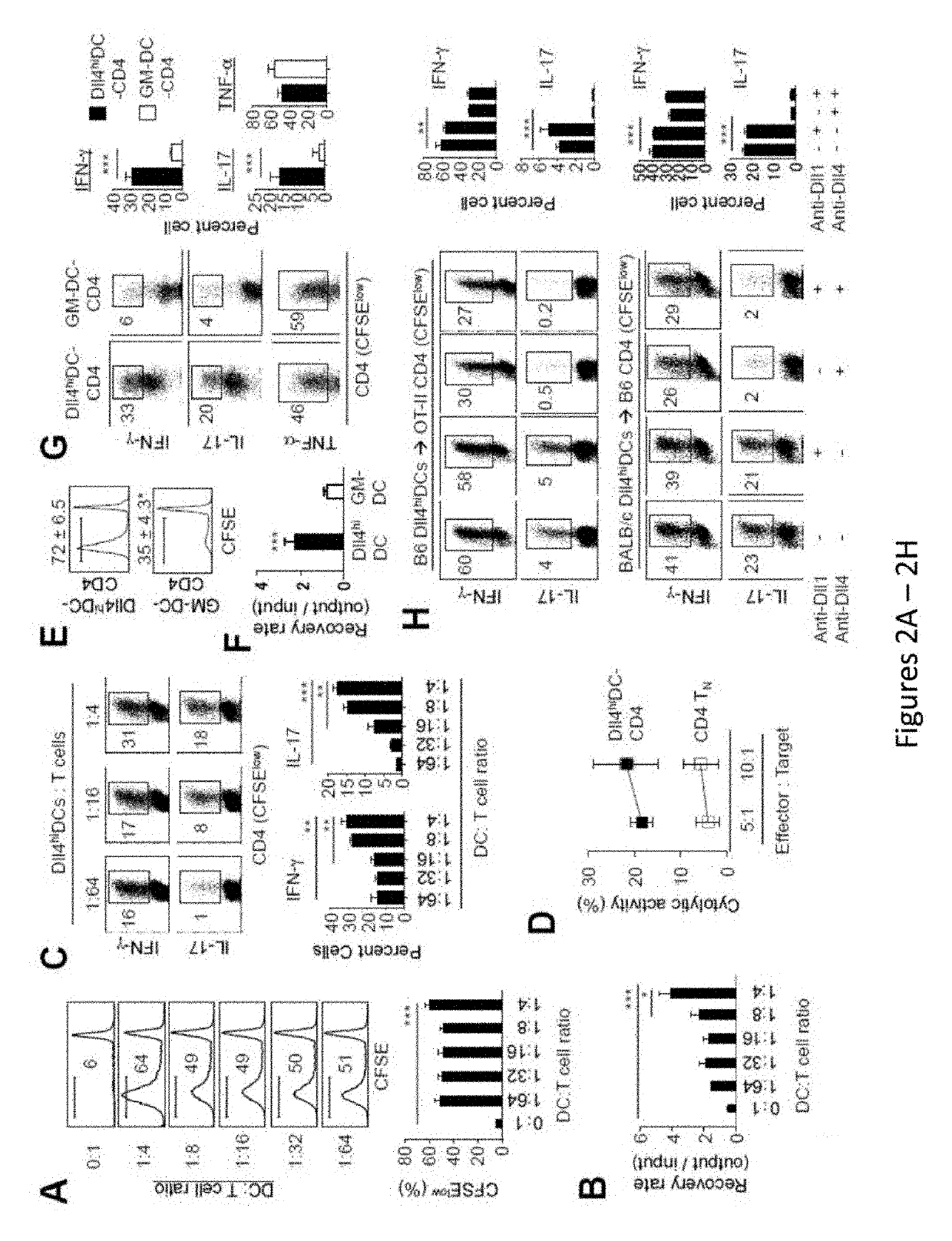
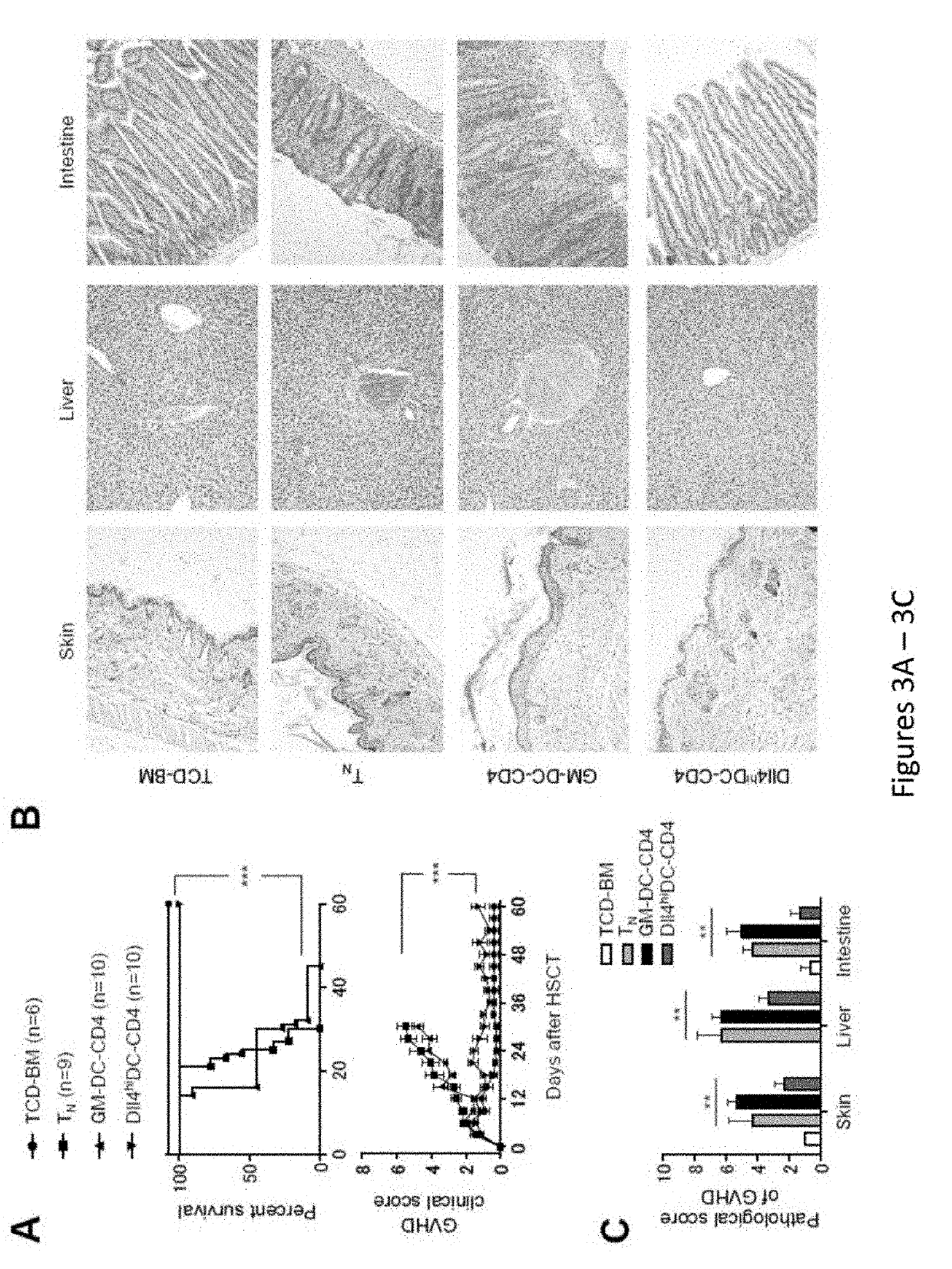
Delta-like 4 is a protein that in humans is encoded by the DLL4 gene. This gene is a homolog of the Drosophila delta gene. The delta gene family encodes Notch ligands that are characterized by a DSL domain, EGF repeats, and a transmembrane domain.
Compositions and methods for modulating vascular development
Owner:GENENTECH INC
Therapeutic dll4 binding proteins
ActiveUS20110117079A1High affinityEliminate needAntibacterial agentsSenses disorderDiseaseTumor angiogenesis
Improved DLL4 binding proteins are described, including antibodies, CDR-grafted antibodies, human antibodies, and DLL4 binding fragments thereof, proteins that bind DLL4 with high affinity, and DLL4 binding proteins that neutralize DLL4 activity. The DLL4 binding proteins are useful for treating or preventing cancers and tumors and especially for treating or preventing tumor angiogenesis, and / or other angiogenesis-dependent diseases such as ocular neovascularization, or angiogenesis-independent diseases characterized by aberrant DLL4 expression or activity such as autoimmune disorders including multiple sclerosis.
Owner:ABBVIE INC
Targeted binding agents directed to dll4 and uses thereof 524
InactiveUS20100196385A1Improve abilitiesEasy to fixAntibacterial agentsSenses disorderDiseaseMonoclonal antibody
The invention relates to targeted binding agents against DLL4 and uses of such agents. More specifically, the invention relates to fully human monoclonal antibodies directed to DLL4. The described targeted binding agents are useful in the treatment of diseases associated with the activity and / or overproduction of DLL4 and as diagnostics.
Owner:MEDIMMUNE LLC
Therapeutic methods for treating vascular eye disorders with DII4 antagonists
InactiveUS20080181893A1Inhibition of developmentEnhanced regrowthSenses disorderPeptide/protein ingredientsDiabetic retinopathyIschemic retinopathy
A therapeutic method for treating ischemic or vascular disorders by administering an agent capable of inhibiting human delta-like ligand 4 (Dll4) activity to a subject in need thereof. In one embodiment, the agent is an anti-Dll4 antibody or antibody fragment capable of inhibiting the binding of Dll4 to a Notch receptor. The method of the invention is useful for treating eye disorders such as ischemic retinopathy, diabetic retinopathy, age related macular degeneration, corneal neovascularization, neovascular glaucoma, or retinopathy of prematurity. The method is also useful or treating ischemic or vascular disorders such as ischemic injury, cerebral ischemia, cardiac ischemia, ischemic conditions affecting the limbs and other organs or tissues, arteriovenous malformations, wound healing, organ or tissue transplantation, placental insufficiency, arterial narrowing and occlusion, atherosclerosis, and systemic or pulmonary hypertension.
Owner:REGENERON PHARM INC
Compositions and methods for modulating vascular development
Owner:GENENTECH INC
DLL4 Signaling Inhibitors and Uses Thereof
InactiveUS20100119526A1Inhibit tumor growthEfficiently disruptedMicrobiological testing/measurementImmunoglobulins against cell receptors/antigens/surface-determinantsAngiogenesis growth factorAntigen binding
DLL4-binding antibodies, specifically antibodies preventing Notch signaling and internalization of DLL4, can: more efficiently than inhibitors only preventing DLL4-mediated Notch-signaling disrupt angiogenesis and pathological processes including tumor growth.
Owner:BIOINVENT INT AB
Methods of treating autoimmune diseases with dll4 antagonists
ActiveUS20110189200A1Increase the number ofAvoid elevationNervous disorderAntipyreticImmunologic disordersRegulatory T cell
The present invention provides methods of treating a disease or disorder, in which increasing the number of regulatory T cell (Treg) is beneficial, by administering to a subject suffering from such a disease or disorder a therapeutically effective amount of DII4 antagonists that block DII4-Notch signal pathways, thereby increasing the number of Treg. Diseases or disorders treatable by the methods of the invention include autoimmune diseases or disorders, such as multiple sclerosis (MS), diabetes, and the like. Suitable DII4 antagonists for the invention include antibodies or antibody fragments that specifically bind DII4 and block DII4-Notch interactions, the extracellular domain of DII4, and the like. The invention also provides methods of preventing an occurrence or recurrence of such diseases or disorders in a subject predisposed or susceptible to developing such diseases or disorders. Furthermore, the methods of the invention are useful in preventing or treating organ transplant rejections or graft-versus-host disease.
Owner:REGENERON PHARM INC
Therapeutic DLL4 binding proteins
DLL4 binding proteins are described herein, including antibodies, CDR-grafted antibodies, humanized antibodies, and DLL4 binding fragments thereof, proteins that bind DLL4 with high affinity, and DLL4 binding proteins that neutralize DLL4 and / or VEGF activity. The DLL4 binding proteins are useful for treating or preventing cancers and tumors and especially for treating or preventing tumor angiogenesis.
Owner:ABBVIE INC
Methods and Monitoring of Treatment with a DLL4 Antagonist
InactiveUS20140227252A1BiocideImmunoglobulins against cell receptors/antigens/surface-determinantsAnticarcinogenSide effect
Methods for treating diseases such as cancer comprising administering a DLL4 antagonist, either alone or in combination with other anti-cancer agents, and monitoring for cardiovascular side effects and / or toxicity.
Owner:ONCOMED PHARMA
Methods of treating diseases with dll4 antagonists
ActiveUS20110189176A1Increase the number ofImprove the level ofNervous disorderAntipyreticDiseaseRegulatory T cell
The present invention provides methods of preventing, treating or ameliorating diabetes by administering to a subject in need thereof a therapeutically effective amount of Dll4 antagonists that block Dll4-Notch signal pathways. As observed in a mouse model of diabetes, Dll4 antagonists exhibit protective effects on pancreatic islets, lower blood glucose levels, and block the production of auto-antibodies, including those against insulin and glutamic acid decarboxylase 65 (GAD65), via the expansion of regulatory T cells (Tregs). Thus, the present invention further provides methods of lowering the levels of blood glucose, and / or reducing or blocking the production of auto-antibodies, by administering to a subject in need thereof a therapeutically effective amount of Dll4 antagonists. Suitable Dll4 antagonists for the invention include antibodies or antibody fragments that specifically bind Dll4 and block Dll4-Notch interactions, the extracellular domain of Dll4, and the like.
Owner:REGENERON PHARM INC
Compositions and methods for diagnosing and treating cancer
An isolated antibody that specifically binds to an extracellular domain of human DLL4 and affects growth of a tumor comprising cancer stem cells is described. Also described is a method of treating cancer comprising administering a therapeutically effective amount of an anti-DLL4 antibody.
Owner:ONCOMED PHARMA
Pharmaceutical combinations comprising dual angiopoietin-2 / dll4 binders and Anti-vegf-r agents
InactiveUS20140093498A1Well-tolerable for patientImprove anti-cancer effectOrganic active ingredientsSenses disorderDiseaseAnti vegf
The present invention relates to pharmaceutical combinations comprising dual Angiopoietin-2 / DII4 binders and anti-VEGF-R agents for use in treating diseases like cancer and ocular diseases.
Owner:BOEHRINGER INGELHEIM INT GMBH
Methods of enhancing the response to radiation in tumor therapy using anti-DLL4 antibodies
InactiveUS8685401B2Immunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsTumor therapyNeoplasm
The disclosure provides methods of treating cancer, tumors, and neoplasias by administering ionizing radiation and an anti-DLL4 antibody or a DLL4-binding fragment.
Owner:HARRIS ADRIAN +2
Anti-dll4 antibodies and methods using same.
InactiveCN101490084AEasy accessQuick clearImmunoglobulins against growth factorsAntibody ingredientsAntibodyVirology
Owner:GENENTECH INC
Therapeutic DLL4 Binding Proteins
ActiveUS20160031986A1BacteriaPeptide/protein ingredientsTumor angiogenesisAngiogenesis growth factor
DLL4 binding proteins are described herein, including antibodies, CDR-grafted antibodies, humanized antibodies, and DLL4 binding fragments thereof, proteins that bind DLL4 with high affinity, and DLL4 binding proteins that neutralize DLL4 and / or VEGF activity. The DLL4 binding proteins are useful for treating or preventing cancers and tumors and especially for treating or preventing tumor angiogenesis.
Owner:ABBVIE INC
Methods for Treating Melanoma
ActiveUS20120070438A1Microbiological testing/measurementImmunoglobulins against cytokines/lymphokines/interferonsMetastatic melanomaExtracellular Structure
Methods of inhibiting melanoma tumor growth, methods of treating melanoma and metastatic melanoma, and methods of reducing the frequency of tumor initiating cells (or cancer stem cells) in melanoma tumors are described. The methods described comprise administering a DLL4 antagonist (e.g., an antibody that specifically binds the extracellular domain of human DLL4) to a subject. Related polypeptides and polynucleotides, compositions comprising the DLL4 antagonists, and methods of making the DLL4 antagonists are also described.
Owner:MEREO BIOPHARMA 5 INC
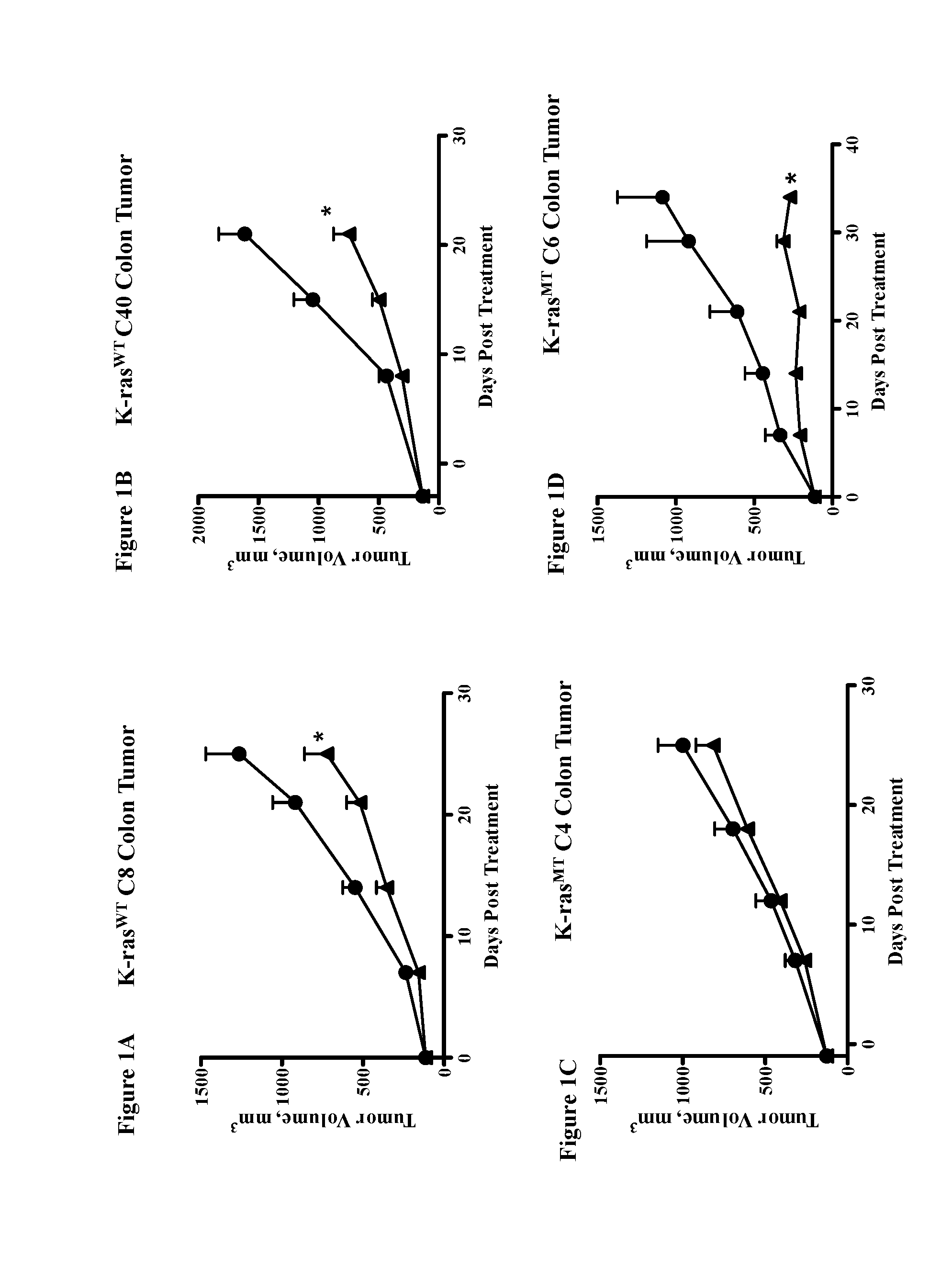
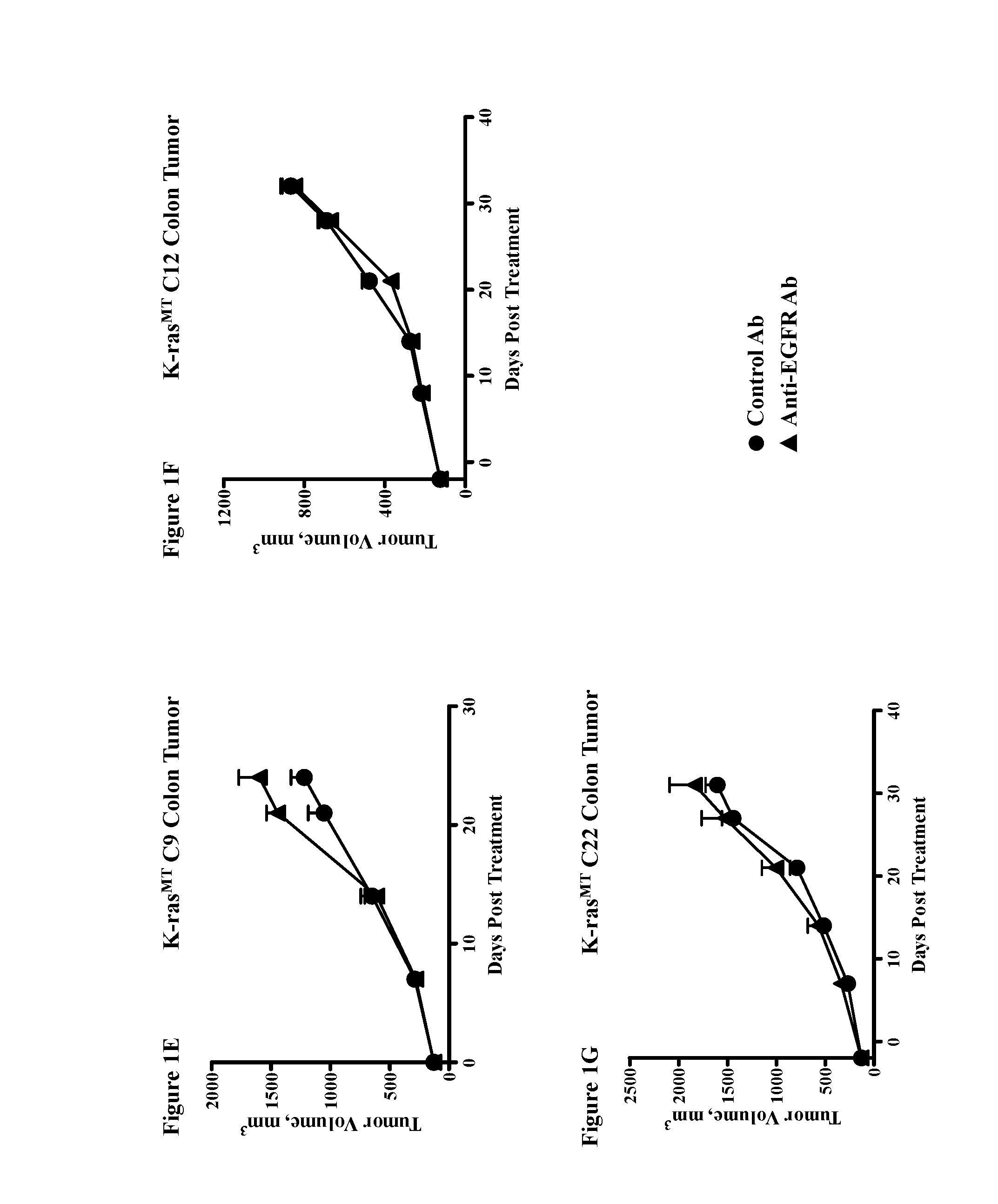
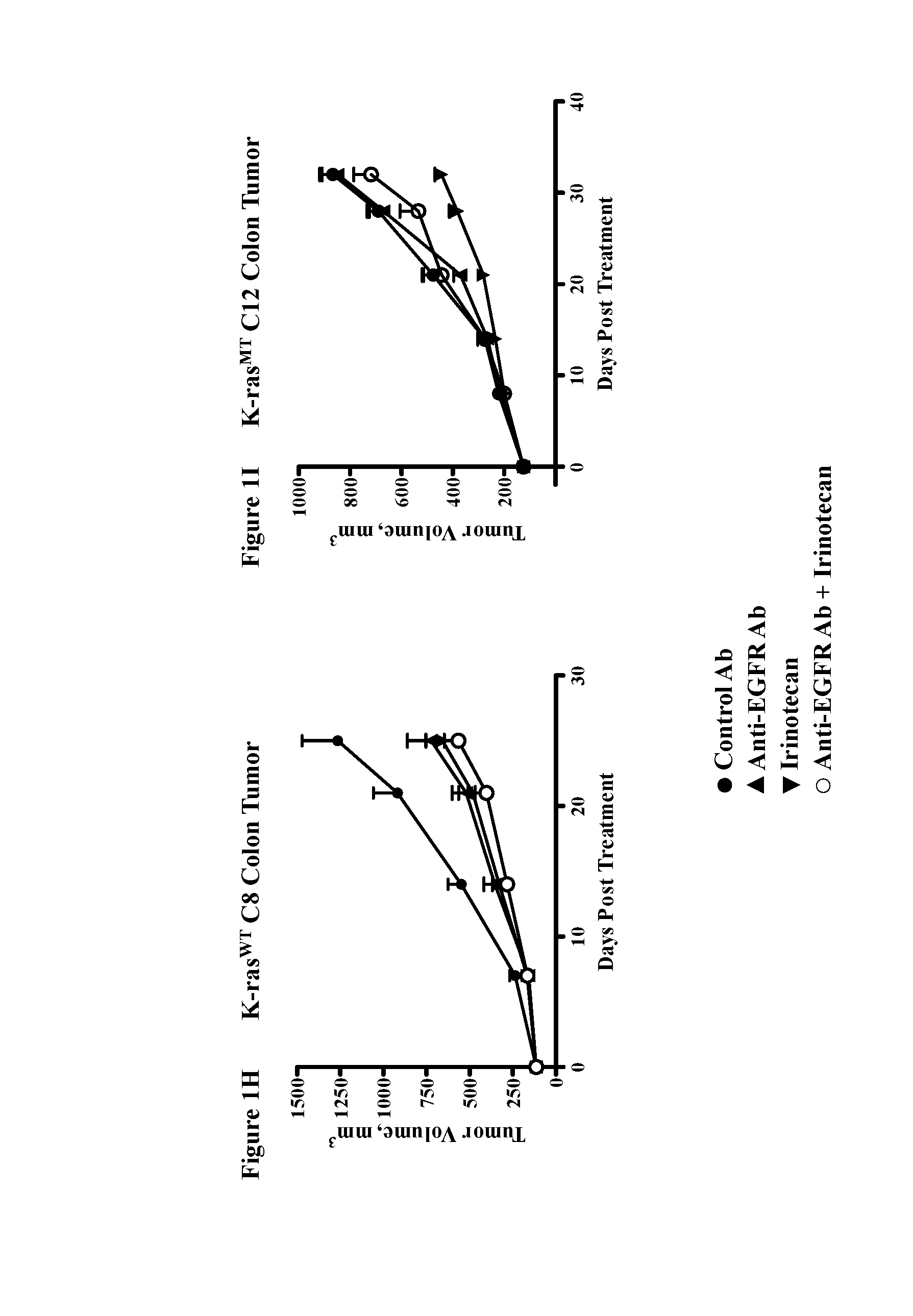
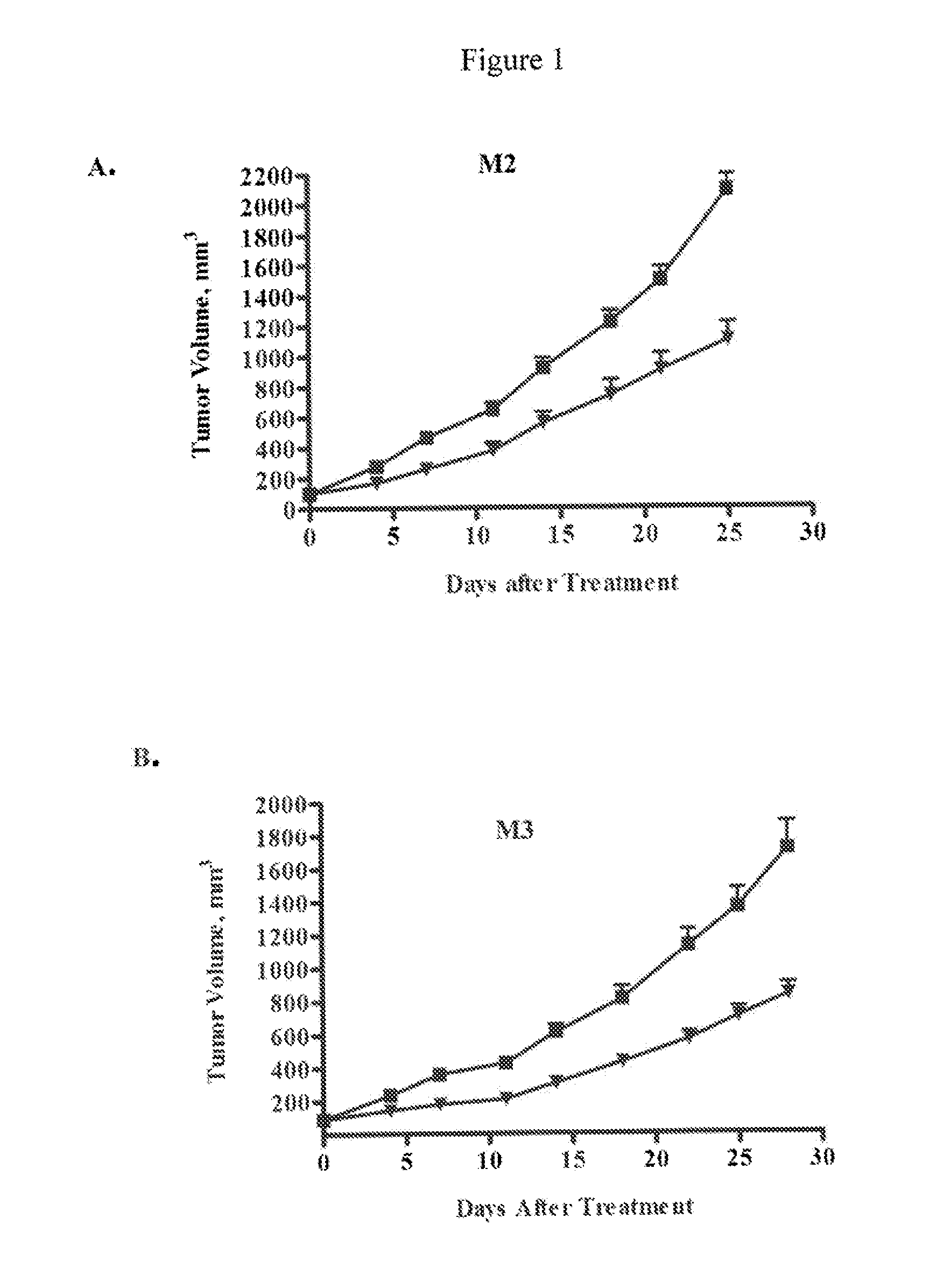
Methods for treating cancers comprising k-ras mutations
InactiveUS20160243223A1Immunoglobulins against growth factorsAntibody ingredientsExtracellular StructureWilms' tumor
Methods of inhibiting tumor growth, methods of treating cancer, and methods of reducing the frequency of cancer stem cells in a tumor are described. Particularly, the methods are directed to tumors or cancers that comprise a K-ras mutation. The methods described comprise administering a DLL4 antagonist (e.g., an antibody that specifically binds the extracellular domain of human DLL4) to a subject. Related polypeptides and polynucleotides, compositions comprising the DLL4 antagonists, and methods of making the DLL4 antagonists are also described.
Owner:ONCOMED PHARMA INC
Methods for treating melanoma
ActiveUS8551479B2Microbiological testing/measurementImmunoglobulins against cytokines/lymphokines/interferonsMetastatic melanomaExtracellular Structure
Methods of inhibiting melanoma tumor growth, methods of treating melanoma and metastatic melanoma, and methods of reducing the frequency of tumor initiating cells (or cancer stem cells) in melanoma tumors are described. The methods described comprise administering a DLL4 antagonist (e.g., an antibody that specifically binds the extracellular domain of human DLL4) to a subject. Related polypeptides and polynucleotides, compositions comprising the DLL4 antagonists, and methods of making the DLL4 antagonists are also described.
Owner:MEREO BIOPHARMA 5 INC
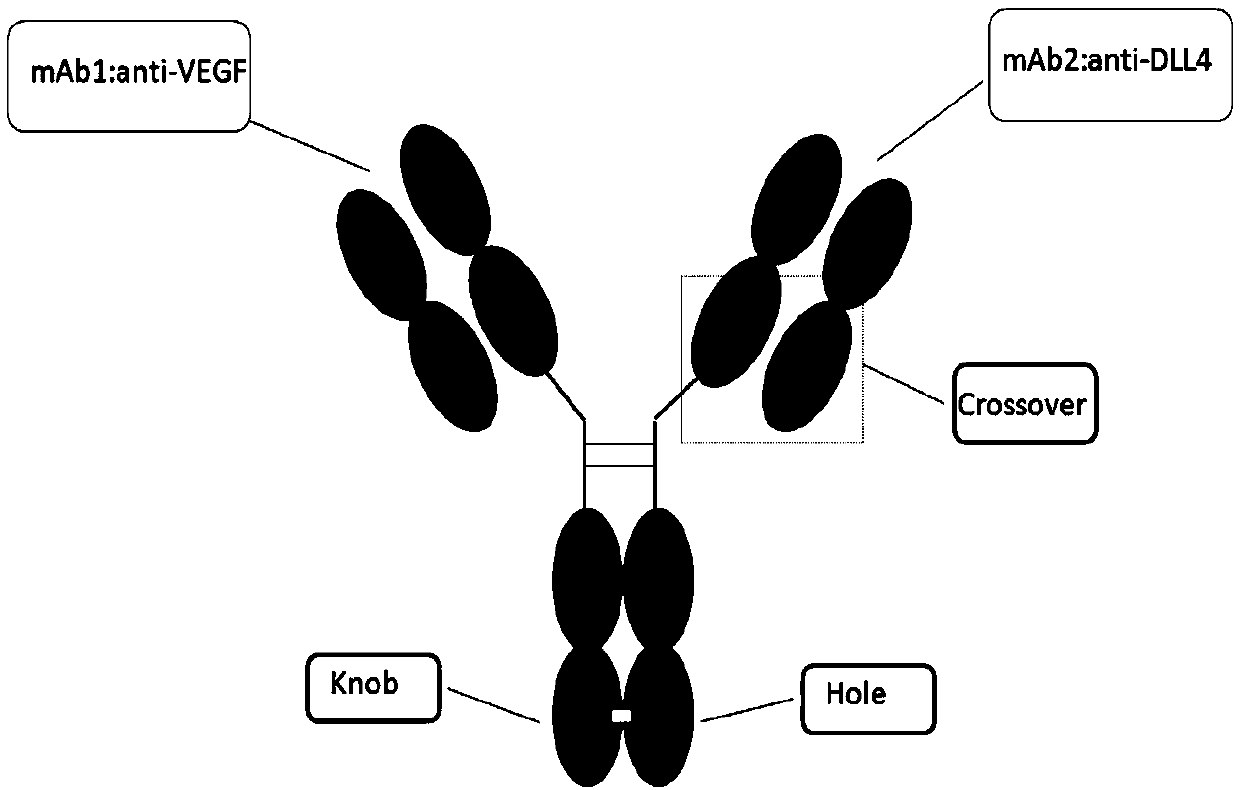
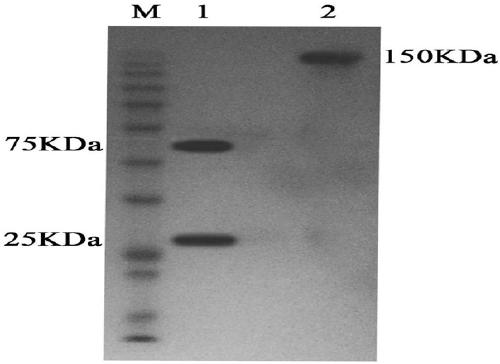
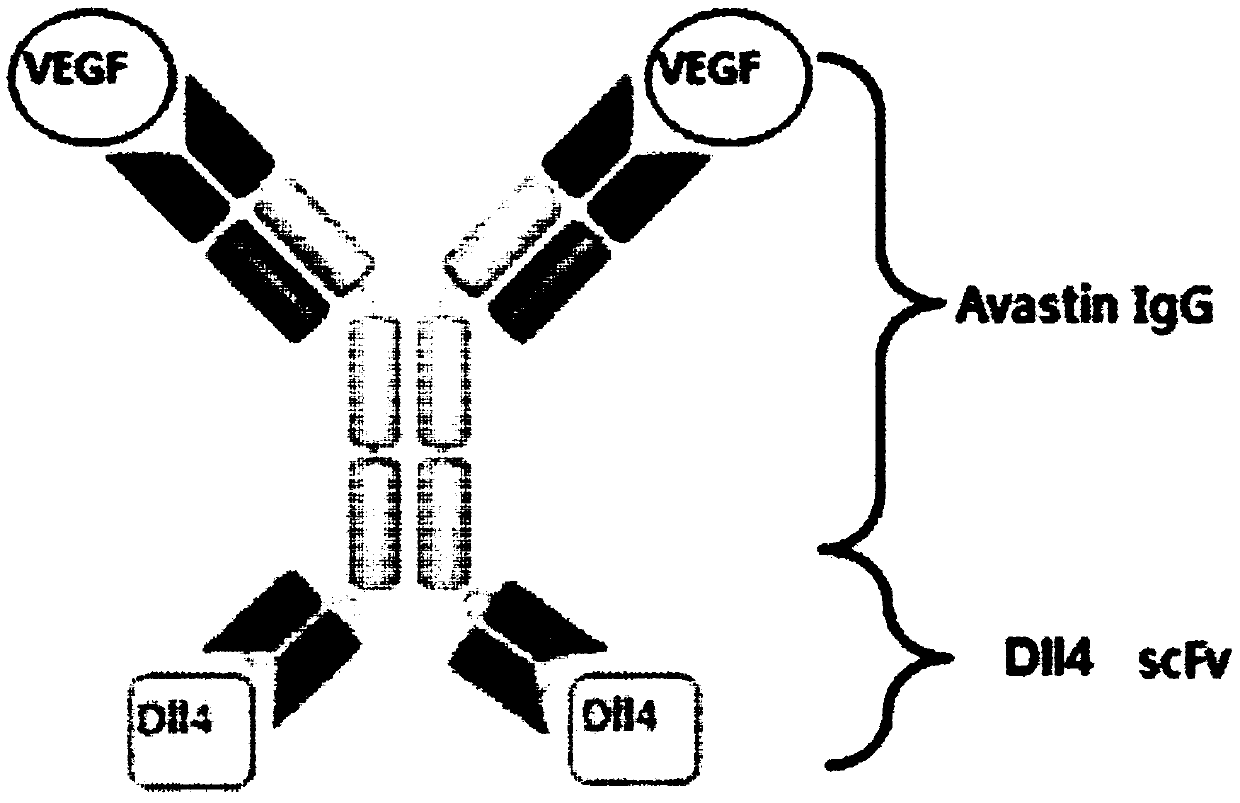
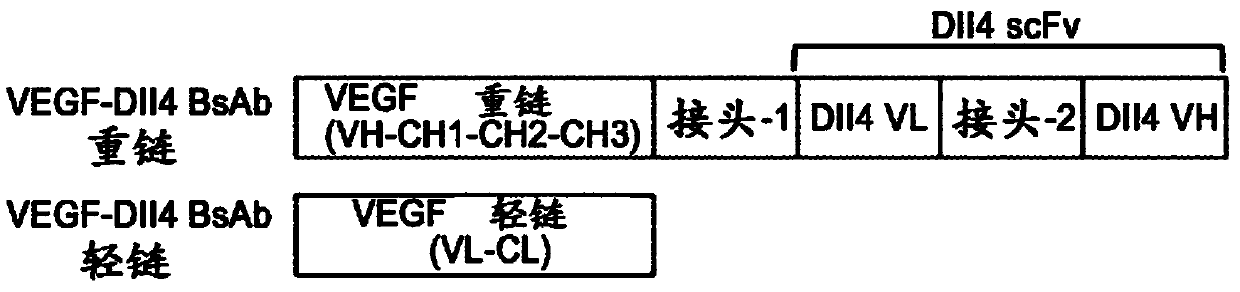
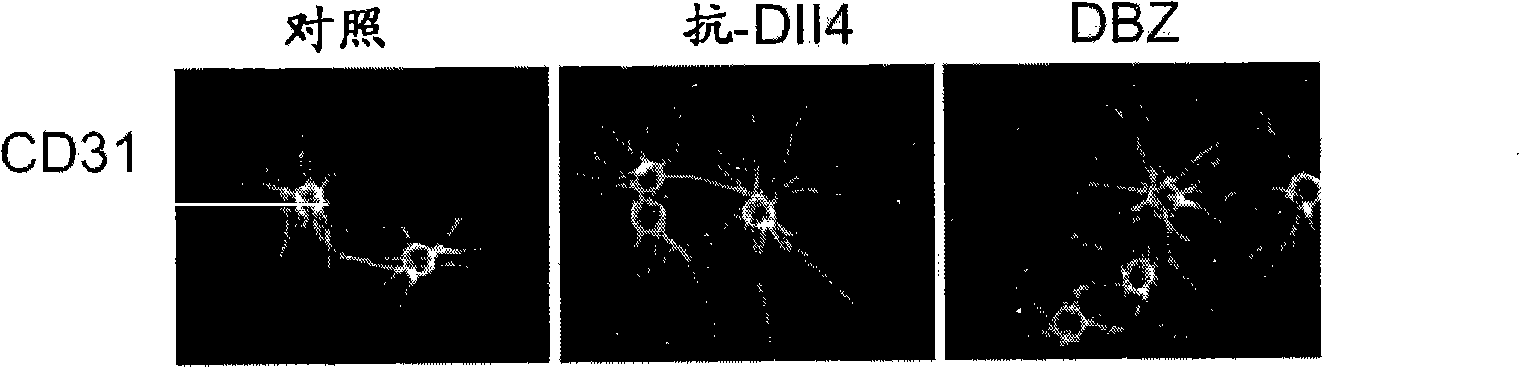
Anti-human DLL4 and VEGF bispecific antibody and preparation and application thereof
ActiveCN109666073AInhibition formationGood antitumor activityHybrid immunoglobulinsImmunoglobulins against growth factorsBispecific antibodySpecific antibody
The invention belongs to the field of antibodies. A bispecific antibody targeting to both VEGF and DLL4 is characterized in that the antibody comprises four chains, namely, an amino acid sequence shown by SEQ ID NO: 1, an amino acid sequence shown by SEQ ID NO: 2, an amino acid sequence shown by SEQ ID NO: 3 and an amino acid sequence shown by SEQ ID NO: 4 in sequence. The dual-targeted VEGF and DLL4 bispecific antibody HB-32 is constructed for the first time, and the antibody can be bound with the antigens VEGF and DLL4 respectively and has good antitumor activity.
Owner:CHINA PHARM UNIV
Novel dual-targeted protein specifically binding to DLL4 AND VEGf, and use thereof
ActiveCN105518028AImprove bindingExcellent strength and anticancer effectNervous disorderSkeletal disorderVascular endotheliumBiology
The present invention relates to a dual-targeted protein comprising: a novel protein specifically binding to delta like ligand 4 (DLL4); and an antibody specifically binding to a vascular endothelial cell growth factor (VEGF).
Owner:ABL BIO INC
Compositions and methods for modulating vascular development
InactiveCN101489576AEasy accessQuick clearPeptide/protein ingredientsAntibody ingredientsAntibodyMolecular biology
Owner:GENENTECH INC
A recombinant baculovirus, a preparing method thereof and applications in preparation of cancer vaccines
InactiveCN104694574AEnhance expressive abilityImprove stabilityGenetic material ingredientsFermentationInfection rateTransmembrane domain
Owner:特菲(天津)生物医药科技有限公司
DLL4-expressing cells and vaccine using the same
InactiveUS20190134169A1Polypeptide with localisation/targeting motifImmunoglobulin superfamilyT cellHost disease
Methods are provided for generating DLL4-expressing immune cells. The invention also includes cellular compositions of dendritic and T cells produced by these methods. The immune cells of the invention can be used widely as components in many diagnostic and therapeutic systems, including improved vaccines to reduce the risk of graft versus host disease.
Owner:TEMPLE UNIVERSITY
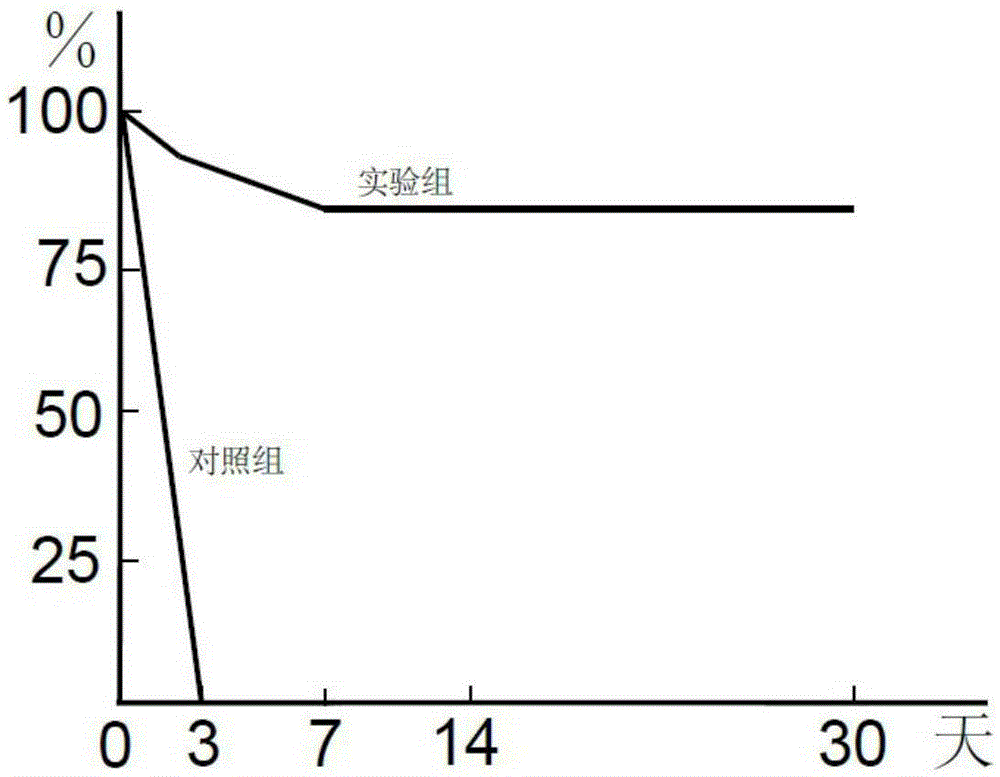
Application of DLL4 cytokine in preparation of medicine for treating fulminant hepatic failure
ActiveCN105267953AReduce degeneration and necrosisInhibit apoptosisPeptide/protein ingredientsDigestive systemFreeze-dryingAntioxidant
The present invention discloses application of DLL4 cytokine in preparation of a medicine for treating fulminant hepatic failure, the medicine includes a DLL4 cytokine pharmaceutically acceptable excipient, an antioxidant and a carrier, the form of medicine can be an injection liquid or frozen dry powder or a suspension, the application temperature of the medicine including the DLL4 cytokine is 15 DEG C-30 DEG C, and the humidity is 45%-75%. Studies on the aspects of biochemical indexes, immunohistochemistry, gene expression level, proteomics and the like show that the DLL4 cytokine can be used in preparation of the medicine for effectively treating fulminant hepatic failure, and different dosages and forms of medicines can be produced by compounding the DLL4 cytokine with different solvents and stabilizers.
Owner:ZHEJIANG UNIV
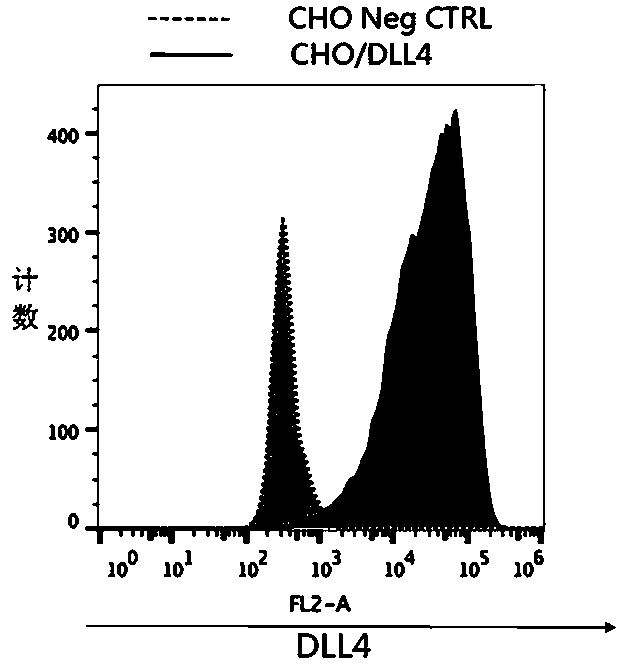
Anti-human DLL4 monoclonal antibody 3F9
ActiveCN107557343AHigh potencyStrong specificityMicroorganism based processesImmunoglobulins against cell receptors/antigens/surface-determinantsMicroorganismMicrobiology
The invention discloses an anti-human DLL4 monoclonal antibody 3F9 which is obtained through secretion of a hybridoma cell strain preserved in CGMCC (China General Microbiological Culture Collection Center) at yard 3, No. 1 Beichen west road, Chaoyang District, Beijing City on June 7, 2017 with the preservation number of CGMCC No.14283. The anti-human DLL4 monoclonal antibody 3F9 is named as hybridoma cell strain 3F9 for secreting the anti-human DLL4 monoclonal antibody. The hybridoma cell strain has the classification and name of hybridoma cell strain 3F9 for secreting the anti-human DLL4 monoclonal antibody. The capability of DLL4+DC for inducing Na-ve T cells to differentiate in the Th1 direction cannot be blocked through binding of the monoclonal antibody 3F9 and DC as compared with commercialized MHD4-46.
Owner:SOOCHOW UNIV AFFILIATED CHILDRENS HOSPITAL
Preparation method of anti-human DLL4 monoclonal antibody 3F9
ActiveCN107556383AHigh potencyStrong specificityMicroorganism based processesImmunoglobulins against cell receptors/antigens/surface-determinantsMicroorganismMicrobiology
The invention discloses a preparation method of an anti-human DLL4 monoclonal antibody 3F9 which is obtained through secretion of a hybridoma cell strain preserved in CGMCC (China General Microbiological Culture Collection Center) at yard 3, No. 1 Beichen west road, Chaoyang District, Beijing City on June 7, 2017 with the preservation number of CGMCC No.14283. The anti-human DLL4 monoclonal antibody 3F9 is named as hybridoma cell strain 3F9 for secreting the anti-human DLL4 monoclonal antibody. The hybridoma cell strain has the classification and name of hybridoma cell strain 3F9 for secretingthe anti-human DLL4 monoclonal antibody. The capability of DLL4+DC for inducing Na-ve T cells to differentiate in the Th1 direction cannot be blocked through binding of the monoclonal antibody 3F9 and DC as compared with commercialized MHD4-46.
Owner:SOOCHOW UNIV AFFILIATED CHILDRENS HOSPITAL
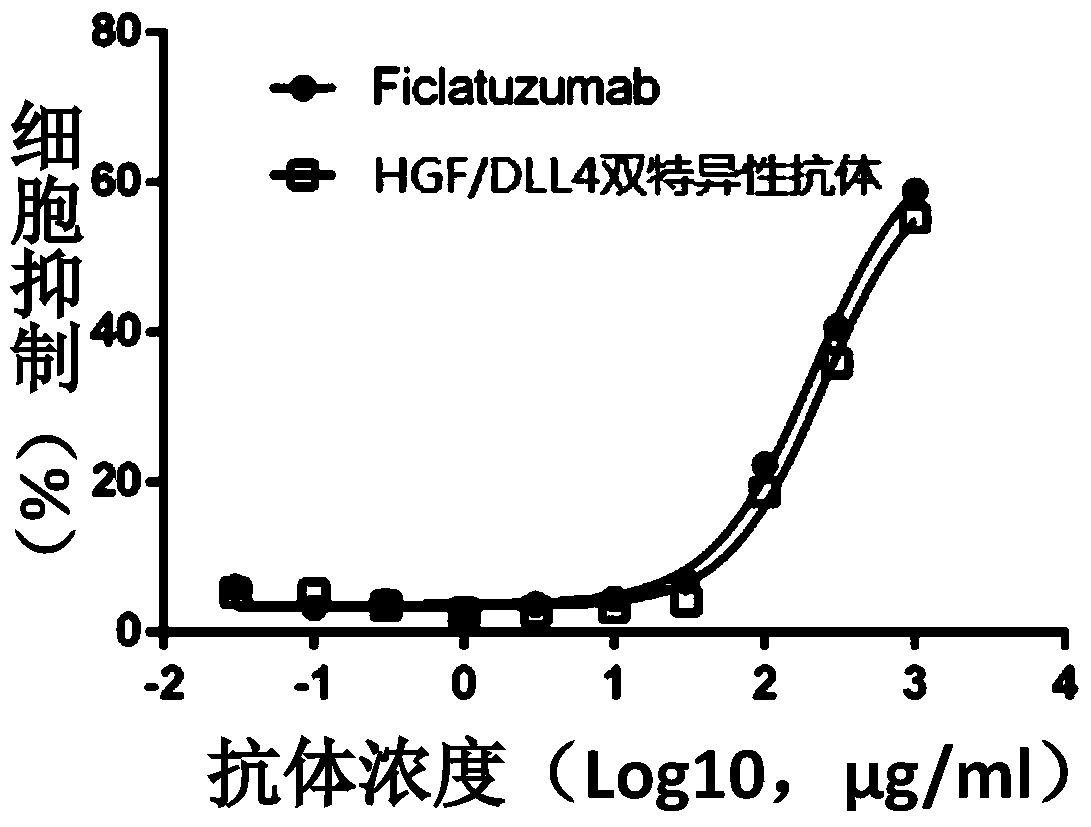
Recombinant anti-hgf/dll4 bispecific antibody, its preparation method and application
ActiveCN104974258BInhibit synthesisHybrid immunoglobulinsAntibody ingredientsBispecific antibodySpecific antibody
The invention belongs to the biotechnical field and particularly discloses a recombinant anti-HGF / DLL4 bispecific antibody and a preparation method and application thereof. The anti-human HGF / DLL4 bispecific antibody can be combined with both HGF and DLL4. The antibody disclosed by the invention has amino acid sequences shown in SEQ ID NO:2 and SEQ ID NO:4. The recombinant anti-human HGF / DLL4 bispecific antibody has an excellent anti-tumor effect.
Owner:SUNSHINE GUOJIAN PHARMA (SHANGHAI) CO LTD
Methods of enhancing the response to radiation in tumor therapy using Anti-dll4 antibodies
InactiveUS20130006034A1Immunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsTumor therapyAntibody
The disclosure provides methods of treating cancer, tumors, and neoplasias by administering ionizing radiation and an anti-DLL4 antibody or a DLL4-binding fragment.
Owner:HARRIS ADRIAN +2
Compositions and methods for diagnosing and treating cancer
An isolated antibody that specifically binds to an extracellular domain of human DLL4 and affects growth of a tumor comprising cancer stem cells is described. Also described is a method of treating cancer comprising administering a therapeutically effective amount of an anti-DLL4 antibody.
Owner:ONCOMED PHARMA
Application of dll4 cytokine in preparation of medicament for treating fulminant hepatic failure
ActiveCN105267953BReduce degeneration and necrosisImprove fibrosisPeptide/protein ingredientsDigestive systemAntioxidantGene expression level
The present invention provides a use of the DLL4 cell factor in preparing a drug for treating fulminant hepatic failure. DLL4 can not only significantly improve the survival rate in a treatment of fulminant hepatic failure, but also significantly improve the survival rate by improving prognosis of a treatment of a severe or terminal stage hepatic disease having a similar underlying cause, disease progression and prognosis.
Owner:ZHEJIANG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com