Recombinant anti-hgf/dll4 bispecific antibody, its preparation method and application
A bispecific antibody and heavy chain technology, applied in the biological field, can solve the problems of regulatory barriers and high cost in the combined application of monoclonal antibodies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1 Gene Construction and Expression of Anti-DLL4 / HGF Bispecific Antibody
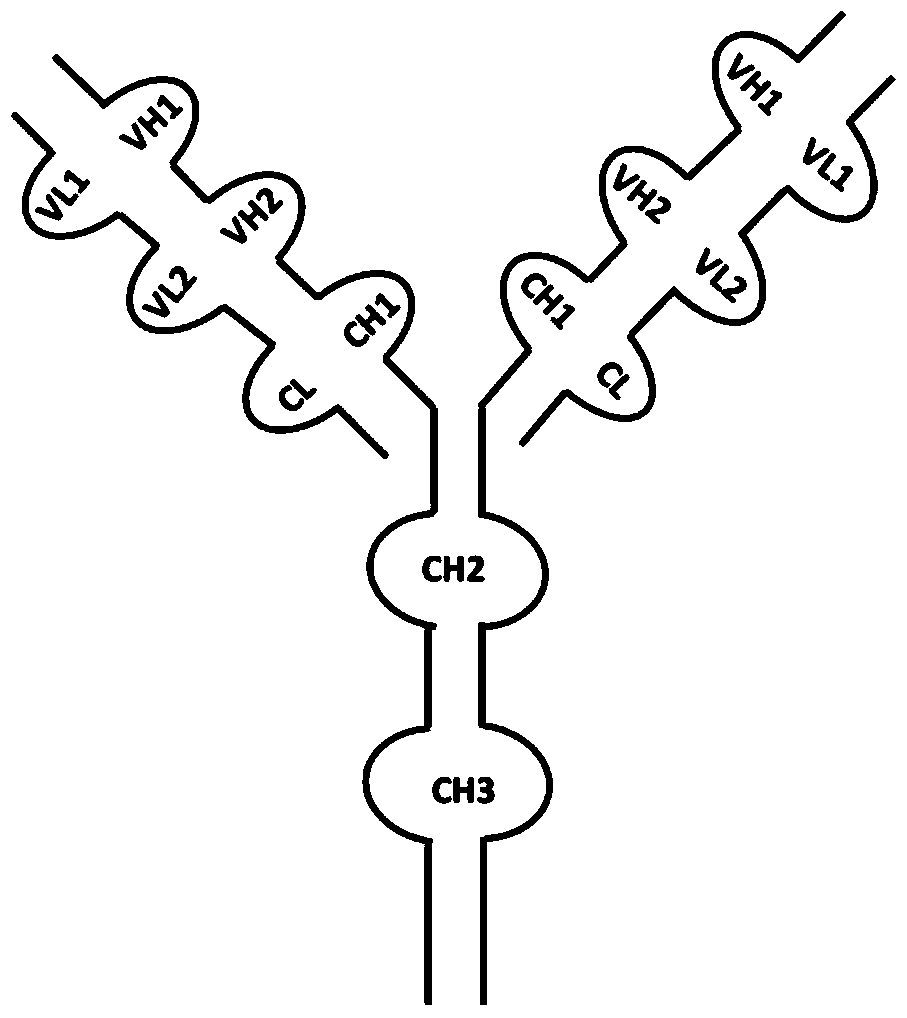
[0047] According to the anti-HGF monoclonal antibody Ficlatuzumab (IgG1, κ) sequence published by who.int (see WHO DrugInformation Vol.25, No.2, 2011.p171), compare the constant region sequence analysis of human IgG1 to obtain its VH and VL fragments . At the same time, according to the amino acid sequence of the anti-DLL4 monoclonal antibody Enoticumab (IgG1, κ) published by who.int (see WHO Drug Information Vol.27, No.1, 2013.p55), the nucleotide sequence was synthesized from the whole gene after codon optimization . The VH fragment of Ficlatuzumab was fused to the N-terminal of the Enoticumab heavy chain through the (ASTKGP) linker by Overlapping PCR; the VL fragment was fused to the N-terminal of the Enoticumab light chain through the (TVAAP) linker. Linker fragments were derived from N-terminal sequences of human IgG1 CH1 or CK, respectively. The primers for heavy chain Overlapping...
experiment example 2
[0049] Experimental example 2 Detection of anti-DLL4 / HGF bispecific antibody by SDS-PAGE and Western-blot
[0050] The purified anti-DLL4 / HGF bispecific antibody, Ficlatuzumab, and Enoticumab were tested for their purity and molecular weight by polyacrylamide gel electrophoresis under non-reducing (6%) and reducing (12%) conditions, respectively, and at the same time by Western-blot Further identify its properties and molecular weight. The Western-blot method is as follows: transfer the gel after electrophoresis to PVDF membrane by electrotransfer method, add HRP-labeled goat anti-human IgG (H+L) after blocking, wash twice with PBST, and finally develop color with DAB method. The results of polyacrylamide gel electrophoresis and Western-blot showed that under reducing conditions, both Ficlatuzumab and Enoticumab presented heavy chains and light chains with molecular weights of about 55KDa and 25KDa, while anti-DLL4 / HGF bispecific antibodies presented as The molecular weight i...
Embodiment 3
[0051] Example 3 Identification of the Binding Ability of Anti-DLL4 / HGF Bispecific Antibody to HGF
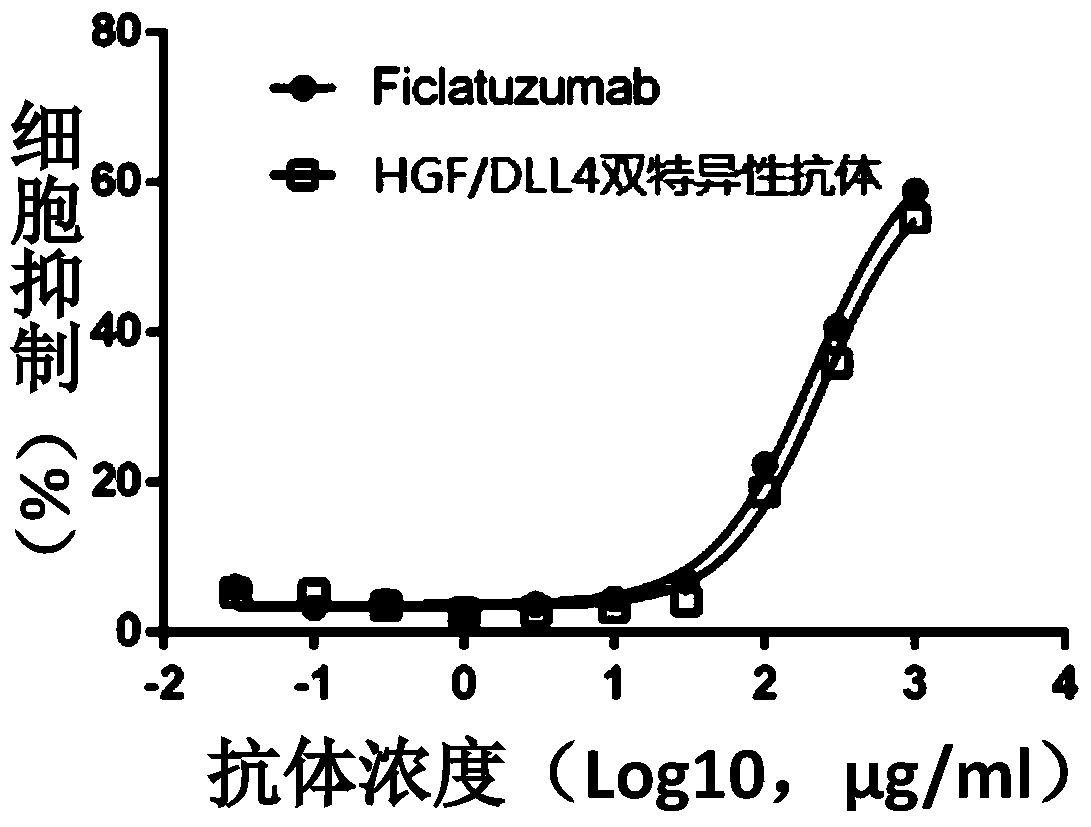
[0052] The binding of the anti-DLL4 / HGF bispecific antibody to HGF was detected by Biacore3000 (purchased from GE). HGF (purchased from R&D Company) was coated on the Biacore3000 chip at different concentrations, and its affinity was detected, and the parent antibody Ficlatuzumab was used as a control. The results are shown in Table 1. It can be seen that the affinity of the anti-DLL4 / HGF bispecific antibody to HGF is similar to that of the parent antibody Ficlatuzumab.
[0053] Table 1, Affinity of anti-DLL4 / HGF bispecific antibodies to HGF
[0054]
[0055] The binding activity of the anti-DLL4 / HGF bispecific antibody to HGF was detected by the human umbilical vein endothelial cells (Human Umbilical Vein Endothelial Cells, HUVEC) proliferation assay. Passage HUVECs with good growth status (purchased from: ATCC company) were inoculated into 96-well plates. After 2 days, 5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com