Compositions and methods for diagnosing and treating cancer
A therapeutic agent, antibody technology, applied in the field of oncology, can solve problems such as failure of treatment methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
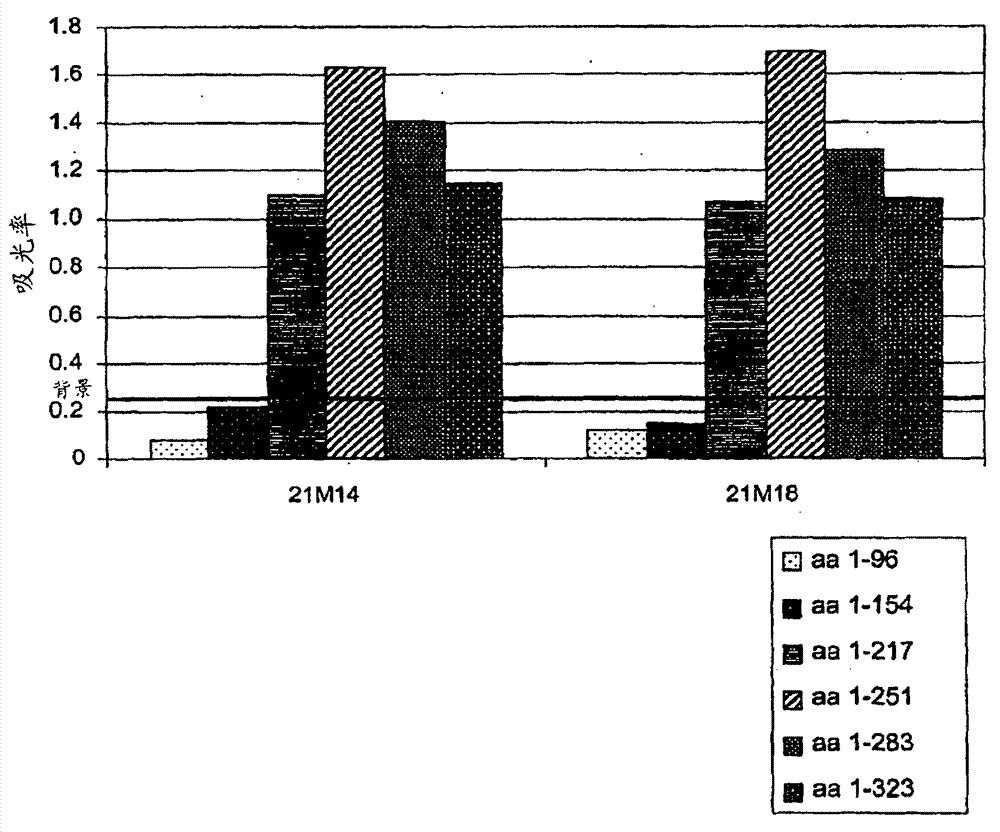
[0183] Example 1 Preparation of monoclonal DLL4 antibody and humanized DLL4 antibody
[0184] Antigen preparation
[0185] The recombinant polypeptide fragment of the extracellular domain of human DLL4 was prepared as an antigen for antibody preparation. The polynucleotide encoding amino acids 1-522 of DLL4 (SEQ ID NO: 25) was isolated using standard recombinant DNA techniques. The polynucleotide is N-terminally linked in frame to a human Fc tag or a histidine tag and cloned into a transfer plasmid vector for baculovirus-mediated expression in insect cells. Standard transfection, infection, and cell culture procedures are used to generate recombinant insect cells expressing the corresponding DLL4 polypeptides (O'Reilley et al., Baculovirus expression vectors: A Laboratory Manual, Oxford: Oxford University Press (1994)).
[0186] Cleavage of the endogenous signal sequence of human DLL4 was simulated using the cleavage prediction software SignalP 3.0, but the actual in vivo ...
Embodiment 2
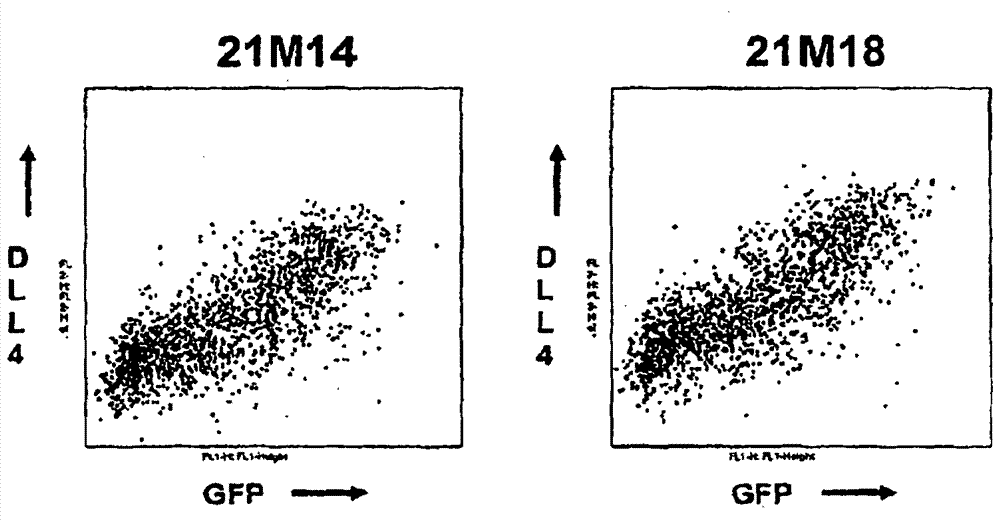
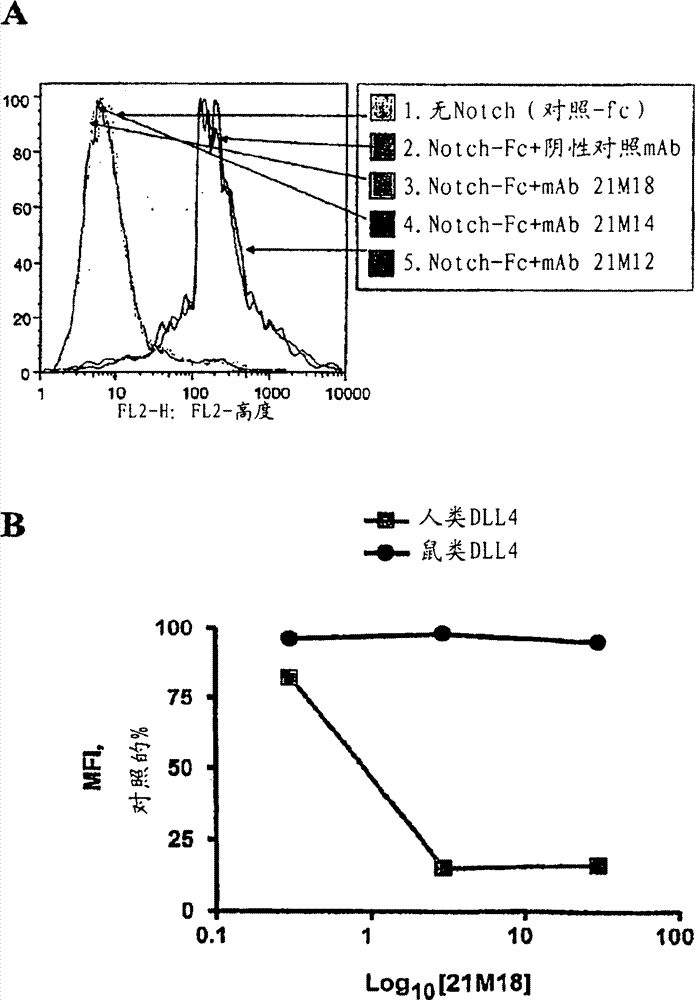
[0211] Example 2: In vitro assays to evaluate antibodies against DLL4
[0212] This example describes a representative in vitro assay for testing the activity of antibodies raised against DLL4 on cell proliferation, Notch pathway activation, and cytotoxicity.
[0213] Proliferation assay
[0214] Taqman analysis was used to quantify the expression of DLL4 in different cancer cell lines. In 96-well tissue culture microplates, 10 4 The density of cells / well will be identified as the DLL4-expressing cell line is plated and allowed to expand for 24 hours. Cells were then cultured for a further 12 hours in fresh DMEM containing 2% FCS, at which time antibodies against DLL4 and control antibodies were added to the medium in the presence of 10 μmol / LBrdU. After BrdU labeling, the medium was removed, cells were fixed in ethanol for 30 minutes at room temperature, and reacted with peroxidase-conjugated anti-BrdU monoclonal antibody (BMG 6H8 clone, Fab fragment) for 90 minutes. Th...
Embodiment 3
[0223] Example 3: Prevention of tumor growth in vivo using antibodies against DLL4
[0224] This example describes the use of anti-DLL4 antibodies to prevent tumor growth in a xenograft model. In certain embodiments, tumor cells that have been passaged in mice as xenografts from patient samples (eg, solid tumor biopsies or pleural effusions) are prepared for passage into experimental animals. Tumor tissue was removed under sterile conditions, cut into small pieces, completely minced with a sterile blade, and single-cell suspension was obtained by enzymatic digestion and mechanical disruption. Specifically, pleural effusion cells or the resulting tumor mass were mixed with ultrapure collagenase III in culture medium (200-250 units collagenase / mL) and incubated at 37°C for 1-4 hours, every 15-20 minutes Pipette up and down through a 10 mL pipette. Digested cells were filtered through 40 [mu]M nylon mesh and washed with Hank's buffered saline solution (HBSS) (pH 7.4) containing...
PUM
| Property | Measurement | Unit |
|---|---|---|
| affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com