Coumarin-porphyrin schiff base compounds and preparation method
A porphyrin Schiff base and compound technology, applied in the field of coumarin-porphyrin Schiff base compound and its preparation, can solve the problem that tetraphenylporphyrin does not have special groups, is not conducive to large-scale synthesis, and has application prospects Limitation and other issues, to avoid the separation process of column chromatography, facilitate large-scale synthesis, and improve the effect of reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
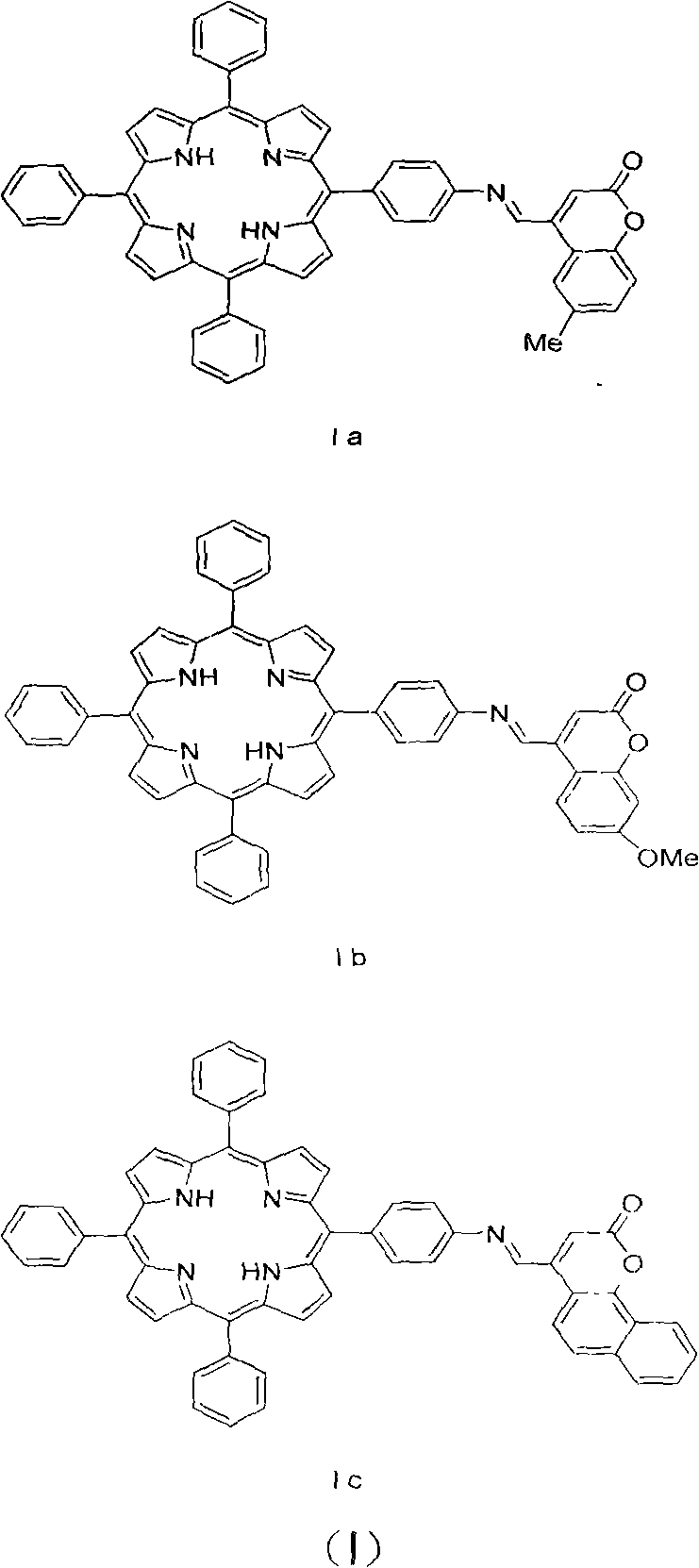
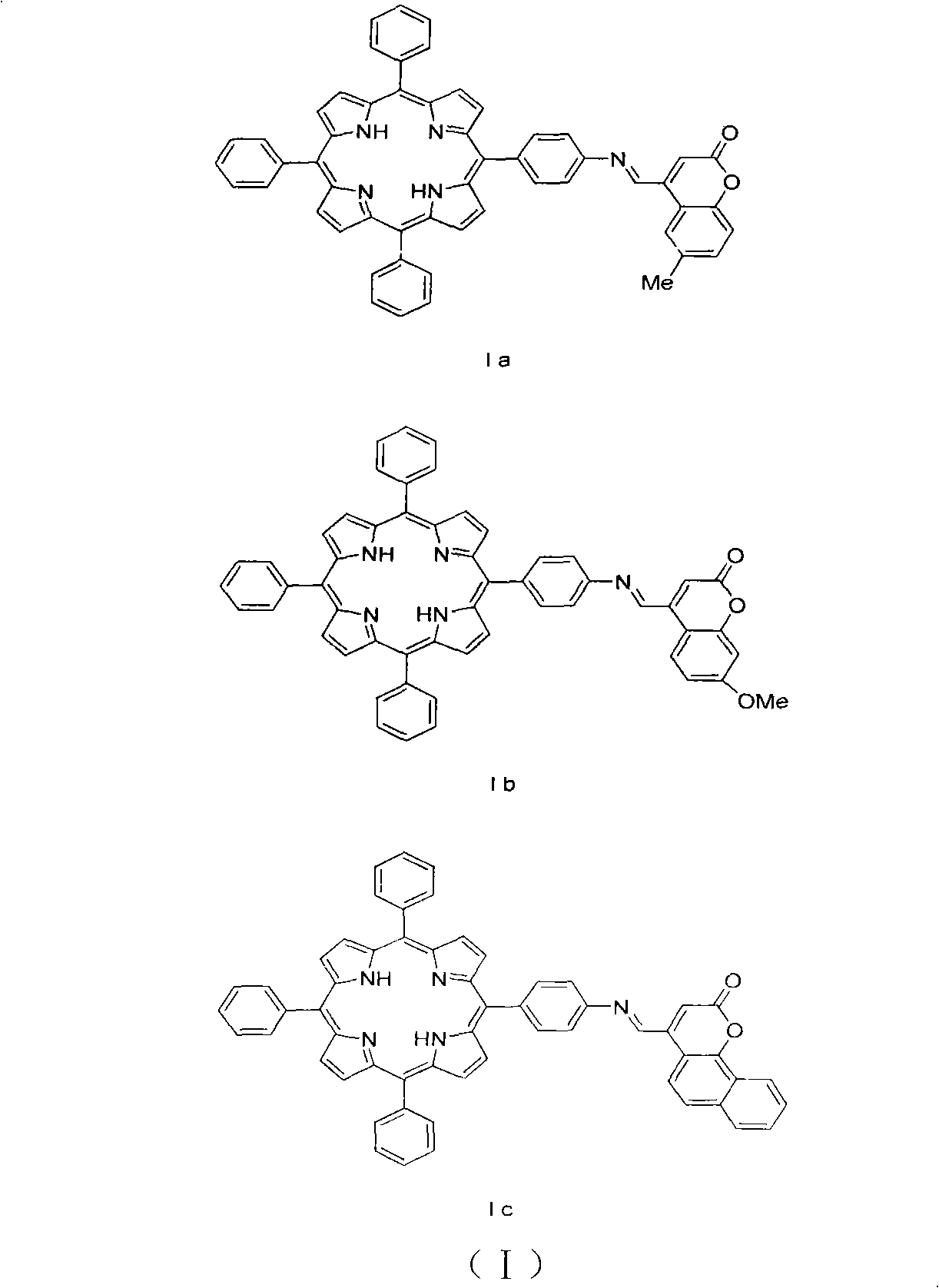
[0018] Example 1: Preparation of 5-[4-(6-methyl-2-oxo-2H-benzopyran-4-methylimino)phenyl]-10,15,20-triphenylporphyrin (Ia)
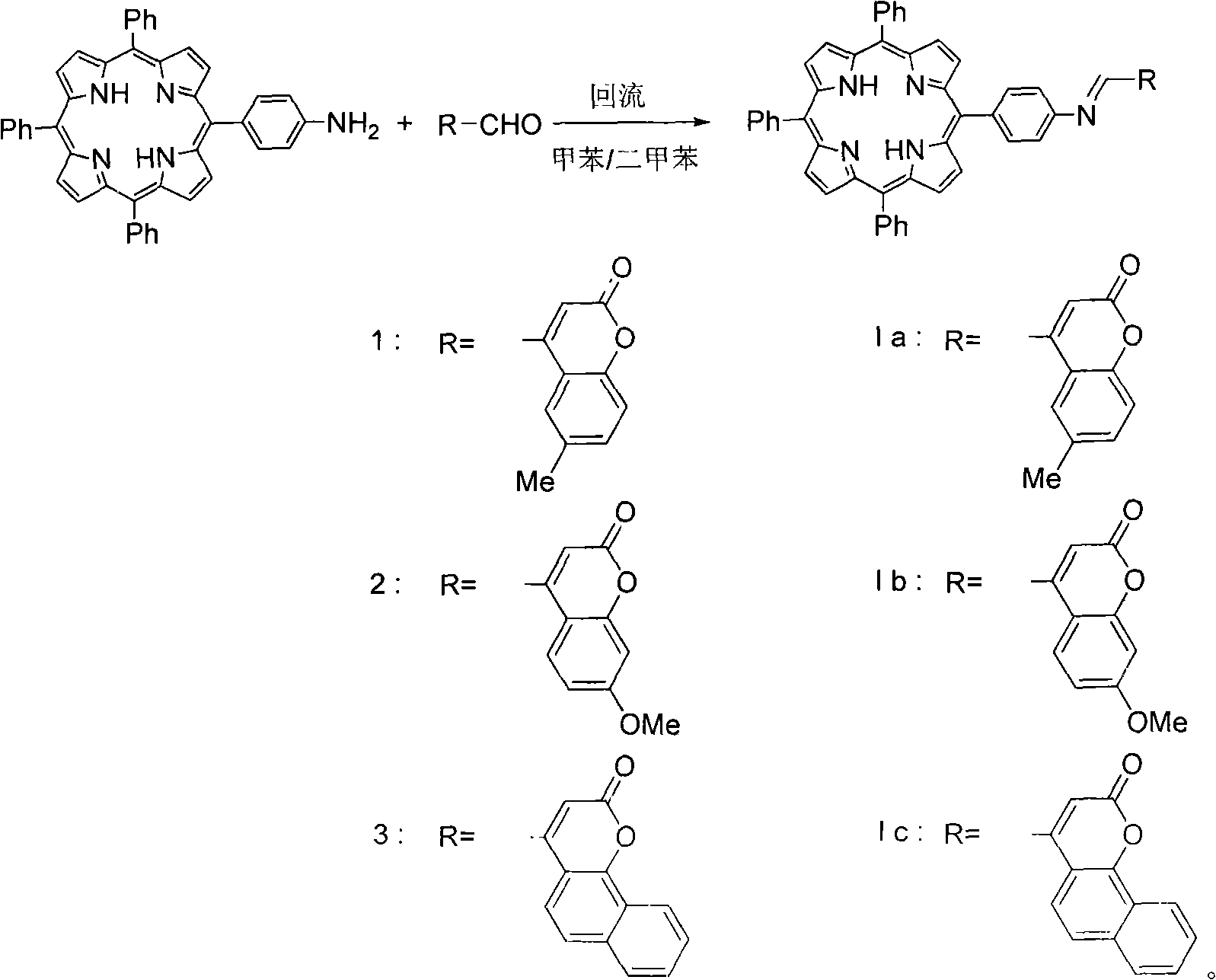
[0019] Take 17mg (0.09mmol) of coumarin aldehyde 1 and 15mg (0.02mmol) of 5-(4-aminophenyl)-10,15,20-triphenylporphyrin in a 50mL round bottom bottle, under nitrogen protection , add processed 15mL toluene or dimethylbenzene, heat to reflux, thin plate chromatography detects, after the raw material reaction is complete, steam the toluene, use methanol / chloroform 5 / 1 (v / v) recrystallization, obtain product (Ia ) 16 mg, yield 71%. Its characterization: melting point > 300 ° C; NMR (CDCl 3 , 500MHz, δppm): -2.77 (s, 2H, NH in the porphyrin ring), 2.55 (s, 3H, methyl H), 7.03 (s, 1H, 3-position H of coumarin), 8.37 (d, J =9Hz, 1H, 8-position H of coumarin), 7.47~7.51(m, 1H, 8-position H of coumarin), 7.72~7.78(m, 11H, para- and meta-11H of porphyrin and 5-position of coumarin H), 7.92~8.04(m, 2H, porphyrin ortho-H), 8.23~8.26(m, 6H, porphyrin ortho-H), 8....
example 2
[0022] Example 2: 5-[4-(7-methoxy-2 oxygen-2H-benzopyran-4-methylimino)phenyl]-10,15,20-triphenylporphyrin (Ib) preparation
[0023] The coumarin aldehyde 1 in Example 1 is replaced by coumarin aldehyde 2, and 17mg (0.08mmol) coumarin aldehyde 2 and 15mg (0.02mmol) of 5-(4-aminophenyl)-10,15 are added, 20-triphenylporphyrin is in a 50mL round bottom bottle, and other operations are the same as in Example 1. Finally generate 5-[4-(7-methoxy-2 oxygen-2H-benzopyran-4-methylimino)phenyl]-10,15,20-triphenylporphyrin (Ib) 14mg, Yield 62%.
[0024] Melting point > 300°C.
[0025] NMR (CDCl 3 , 500MHz, δppm): -2.75 (s, 2H, NH in the porphyrin ring), 3.96 (s, 3H, methoxy H), 6.97 (d, J=10Hz, 1H, 8-position H of coumarin), 7.02~7.06(d, 1H, 6-position H of coumarin), 7.47~7.51(m, 1H, 8-position H of coumarin), 7.70~7.82(m, 11H, porphyrin para-position and meta-position 11H and coumarin 5-position H), 7.90~8.00 (m, 2H, ortho-position H of porphyrin), 8.22-8.24 (m, 6H, ortho-position...
example 3
[0028] Example 3: Preparation of 5-[4-(2-oxo-2H-naphthopyran-4-methylimino)phenyl]-10,15,20-triphenylporphyrin (Ic)
[0029] In example 1, coumarin aldehyde 1 is replaced by coumarin aldehyde 3, and 17mg (0.08mmol) coumarin aldehyde 3 and 15mg (0.02mmol) of 5-(4-aminophenyl)-10,15,20 are added -Triphenylporphyrin 1 is in the 50mL round bottom bottle, and other operations are all the same as example 1. Finally, 5-[4-(2-oxo-2H-naphthopyran-4-methylimino)phenyl]-10,15,20-triphenylporphyrin (Ic) was generated with a yield of 62%.
[0030] Melting point > 300°C.
[0031] NMR (CDCl 3 , 500MHz, δppm): -2.75 (s, 2H, NH in the porphyrin ring), 7.72~7.82 (m, 15H, porphyrin ortho-para H and coumarin 3, 5, 6, 8 H), 7.97~ 8.05(m, 2H, porphyrin ortho-H), 8.22~8.24(m, 7H, porphyrin ortho-H and coumarin 7-position H), 8.68~8.75(m, 2H, coumarin 9, 10-position H ), 8.86~8.90 (m, 8H, 2-position H of porphyrin), 8.945 (s, 1H, -C=NH position H).
[0032] ESI-MS m / z 836.4(M+H) + (calcd.for C ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com