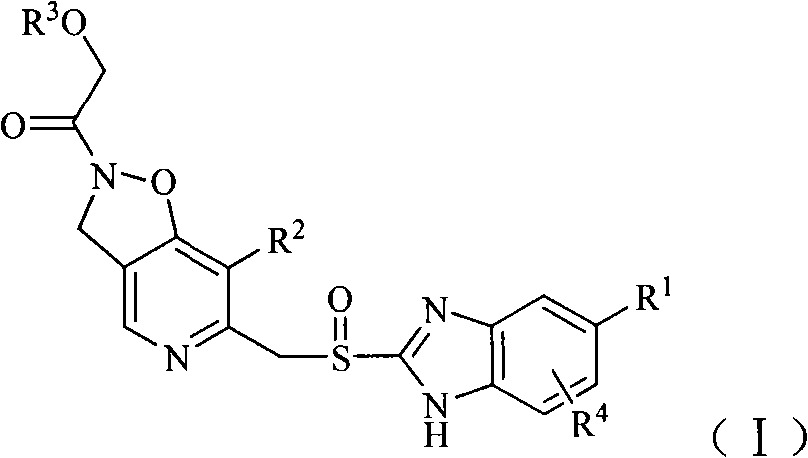
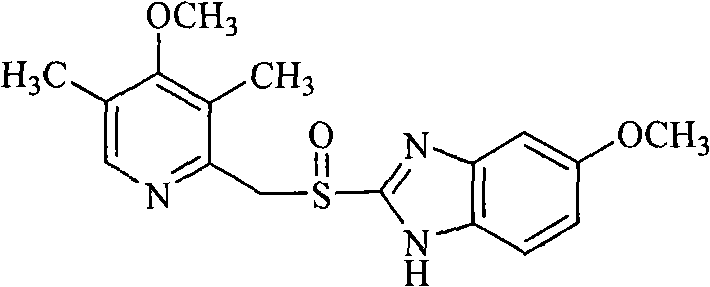
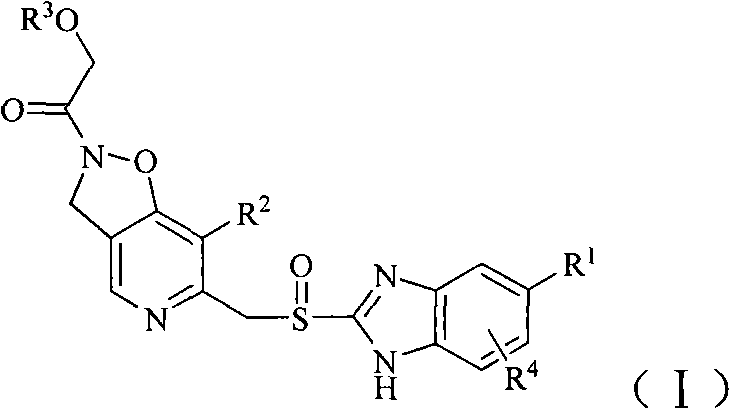
Compound containing alcoxyl acetyl dihydrogen isoxazole-pyridine
A compound, ethyl technology, applied in the field of medicine, can solve problems affecting drug efficacy and pharmacokinetic parameters, insufficient drug effect, slow onset time, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0103] The preparation of embodiment 1 2-mercapto-benzimidazole
[0104] Put 6.5g (60mmol) of phthalic diamine into the reaction flask, add 200ml of 95% ethanol solution, then add 12.8g (80mmol) of potassium ethoxysulfonate, heat and reflux at 80°C for 4h. To room temperature, pour the reaction solution into 200ml of ice water, stir evenly, adjust the pH to 3-4 with 4N hydrochloric acid, precipitate a solid, filter, wash with water until neutral, and vacuum-dry the filter cake to obtain 7.2g of the product, yield: 79.7% .
Embodiment 2
[0105] Example 2 Preparation of 2-mercapto-5-methoxy-benzimidazole
[0106] Preparation method Referring to Example 1, 8.3 g (60 mmol) of 4-methoxy o-phenylenediamine and 12.8 g (80 mmol) of potassium ethoxysulfonate were added to obtain 8.2 g of the product, yield: 75.4%.
Embodiment 3
[0107] Example 3 Preparation of 2-mercapto-5-difluoromethoxy-benzimidazole
[0108] Preparation method Referring to Example 1, 10.4 g (60 mmol) of 4-difluoromethoxy o-phenylenediamine and 12.8 g (80 mmol) of potassium ethoxysulfonate were added to obtain 9.5 g of the product, yield: 73.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com