New furyl thioalkanals useful in the flavor industry
A furanyl, methyl technology, applied in the field of compositions or end products derived from said application, can solve problems such as no aldehyde derivatives, no mention or suggestion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-8
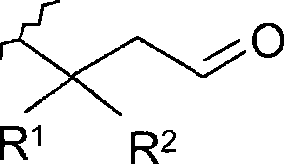
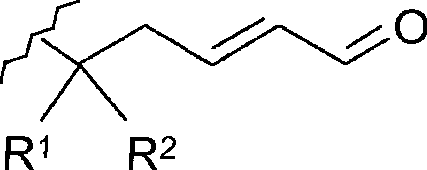
[0065] Compounds of general formula (I) are synthesized by reactions represented in Scheme 1 using appropriate starting materials. compound
[0066] A. Synthesis of 3-[(2-methyl-3-furyl)thio]aldehyde and 3-methyl-3-[(2-methyl-3-furyl)thio]aldehyde
[0067]As a typical synthesis, according to the ratio given in Table 2, 2-methyl-3-mercaptofuran (hereinafter referred to as MFT), 2-alkenal, distilled water and ethanol were stirred at room temperature for several hours (24-95h). Then, the reaction mixture was extracted with ethyl acetate (2 times), and the organic layer was washed with saturated NaCl solution. The combined organic layers were washed with Na 2 SO 4 Dried and concentrated. Column chromatography (SiO 2 , heptane / diethyl ether 8:2) to obtain the pure compound.
[0068] Table 2 : The preparation condition of 3-methyl-3-[(2-methyl-3-furyl) thio] aldehyde
[0069] Aldehyde / concentration
mMol MFT
mMol water
ml ethanol
ml Stiring t...
Embodiment 9
[0104] Synthesis of 3-[(2-methyl-3-furyl)thio]butyraldehyde via Maillard Reaction Intermediate
[0105]A. Reaction of cysteine with xylose followed by addition of natural crotonaldehyde
[0106] Excess cysteine (2.4g, 20mmole) with xylose (0.3g, 2mmole) and NaH 2 PO 4 (2 g, 100 mmole) reacted. The ingredients were dry blended with Hydromatrix (amorphous carrier; Varian part 198003) (17 g). In an ASE (rapid solvent extraction) cell from Dionex, water (60-70 ml) was added and the mixture was heated to 150°C. The pressure was adjusted to 100 bar with nitrogen during a static period of 30 min. Natural crotonaldehyde (1.4 g, 20 mmole) was added to the crude flavor product released from the pool (pH 6.5). Prior to the addition of crotonaldehyde, an aliquot representing 10% of the reaction mixture was extracted with pentane containing 1 mg / ml n-octylthiol as an internal standard and analyzed by GC-MS. After addition of crotonaldehyde, the same extraction was performed. I...
Embodiment 10
[0120] 3-[(2-Methyl-3-furyl)thio]butyraldehyde as a flavoring ingredient in flavor spices and other Application usage
[0121] The above compounds were added in the amounts indicated in Table 4 to various flavor compositions (source: Firmenich SA, Geneva, Switzerland) having the type of flavor profile indicated in Table 4. The table summarizes in all cases the positive sensory effect observed as a result of blind evaluation compared to known fragrances without the compound of the invention. Evaluation tests were carried out by blind test with aqueous solutions containing the indicated ingredients in the indicated proportions (MSG stands for monosodium glutamate).
[0122] Table 4: Performance of 3-[(2-methyl-3-furyl)thio]butyraldehyde in various flavor-type perfumes (increasing number of asterisks indicates increasing level of perfume improvement).
[0123] inside the spice
[0124] * In application, relative to the total weight of the perfumed consumer product...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com